FDA Approves FILSUVEZ Topical Gel for Junctional and Dystrophic EB

The U.S. Food and Drug Administration (FDA) approved FILSUVEZ (birch triterpenes) topical gel for the treatment of partial thickness wounds in patients 6 months and older with Junctional Epidermolysis Bullosa (JEB) and Dystrophic Epidermolysis Bullosa (DEB). Chiesi Global Rare Diseases’ FILSUVEZ is the first approved treatment for wounds associated with JEB, a rare, moderate-to-severe form of […]
Laser Hair Removal Helps Reduce Recurrence of Pilonidal Disease in Teens
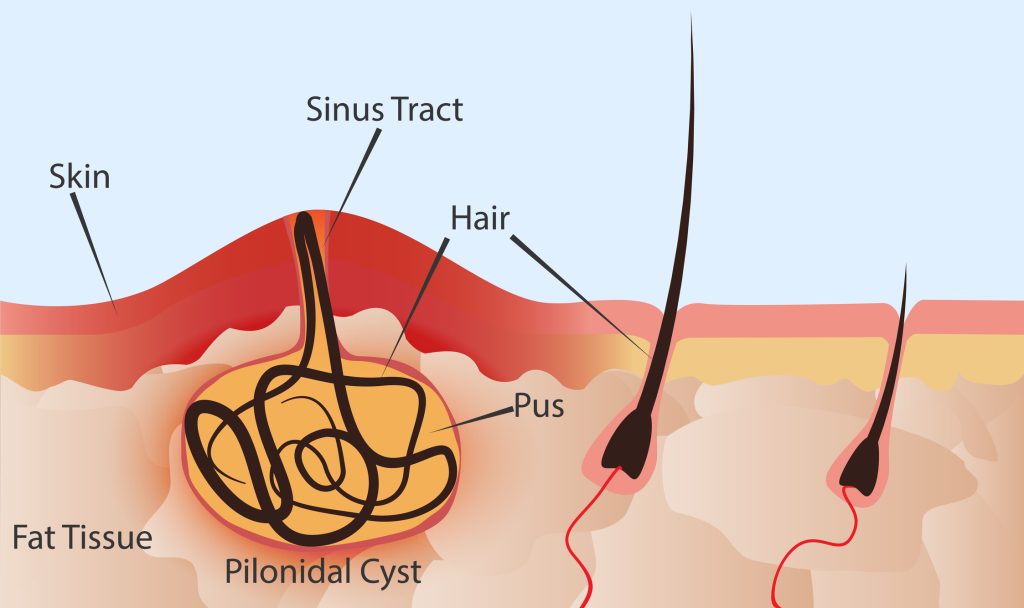
Pilonidal disease is a painful inflammatory disease that occurs when cysts form between the buttocks. These cysts often recur following standard treatment with razors or creams. Peter C. Minneci, MD, Chair of Surgery at Nemours Children’s Health in Delaware Valley, recently conducted a study1 that looked at the use of laser hair removal in these patients. He […]
Proof of Concept: Oral Heat Shock Protein 90 Inhibitor Shows Promise in HS
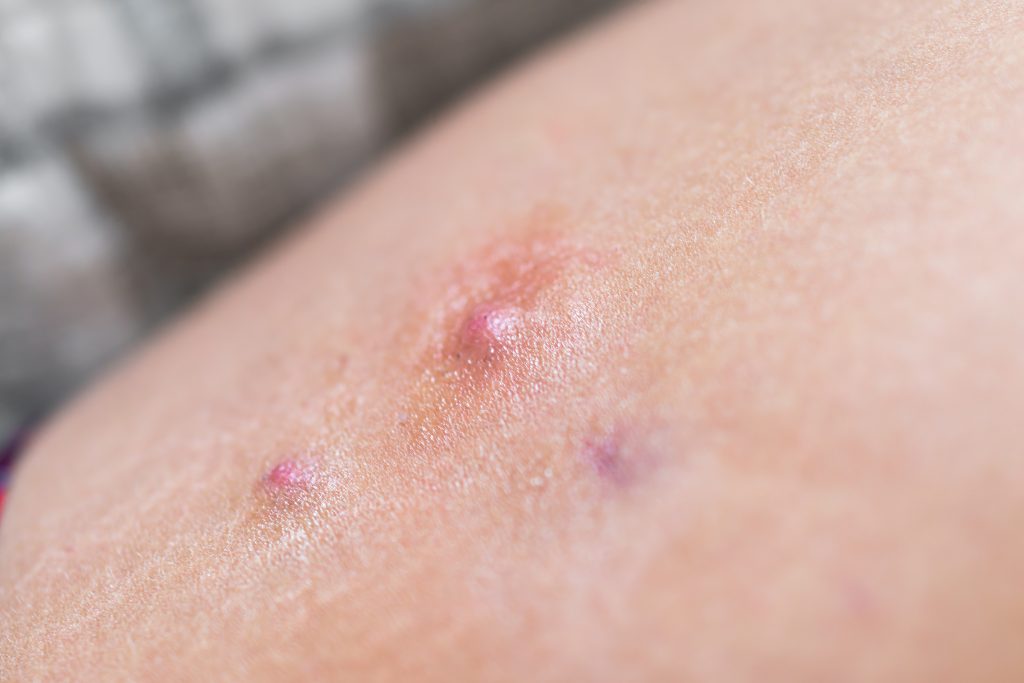
Heat shock protein 90 inhibition by oral RGRN-305 may represent a novel way of treating hidradenitis suppurativa (HS), according to a study in JAMA Dermatology. In the parallel-design double-blind trial of 15 HS patients, those patients who received oral RGRN-305, 250 mg, once daily showed a robust treatment response compared with their counterparts who received placebo after […]
TDD AD Pipeline Watch: Aldeyra’s ADX‑629 Performs Well in Phase 2 Study
Aldeyra Therapeutics’ investigational RASP modulator ADX-629 performed well in a Phase 2 clinical trial of patients with atopic dermatitis (AD), according to topline results. Aldeyra expects to initiate a multicenter, randomized, placebo-controlled Phase 1/2 clinical trial of ADX‑246 in healthy volunteers and patients with AD in the first half of 2024. Topline results from the trial […]
