And Then There Were Two: FDA Approves Zelsuvmi for Molluscum Contagiosum
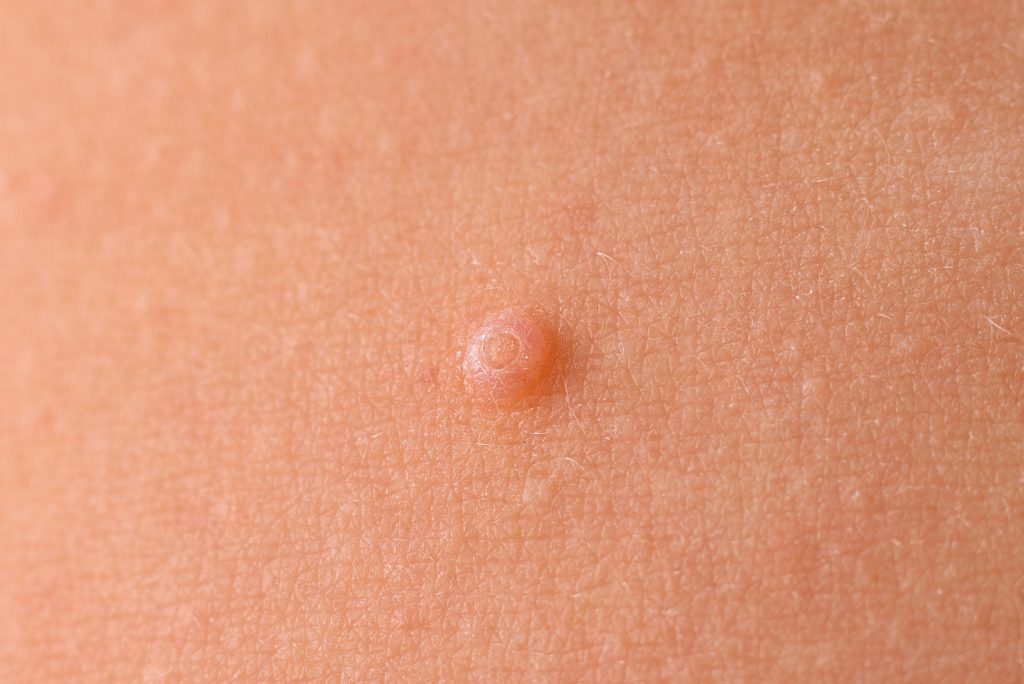
The U.S. Food and Drug Administration (FDA) has approved Zelsuvmi (berdazimer topical gel, 10.3%) for the treatment of molluscum contagiosum in adults and children one year of age and older. Zelsuvmi is expected to be commercially available during the second half of 2024, according to Ligand Pharmaceuticals. Zelsuvmi is the first novel drug for the treatment of molluscum infections […]
Last Patient Dosed in Part 2 of Verrica’s Phase 2 Study of VP-315 in BCC

The last patient has been dosed in Part 2 of Verrica Pharmaceuticals Inc.’s Phase 2 trial of VP-315, a potential first-in-class oncolytic peptide, for the treatment of basal cell carcinoma. VP-315 is a chemotherapeutic administered intratumorally. It works by inducing lysis of intracellular organelles of tumor cells such as mitochondria, thereby unleashing a broad spectrum of […]
Rosacea Pipeline Watch: Journey Medical Submits NDA for DFD-29
Journey Medical Corporation has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) seeking approval for DFD-29 (Minocycline Hydrochloride Modified Release Capsules, 40 mg) for the treatment of inflammatory lesions and erythema of rosacea in adults. DFD-29 is being developed in collaboration with Dr. Reddy’s Laboratories Ltd. “Based on the […]
