FDA, EMA Accept Galderma’s BLA for Nemolizumab for PN and AD

The U.S. Food and Drug Administration (FDA) has accepted Galderma’s Biologics License Applications for nemolizumab for the treatment of patients with prurigo nodularis (PN) and for adolescents and adults with moderate to severe atopic dermatitis (AD). In addition, the European Medicines Agency has also accepted the Marketing Authorization Applications for nemolizumab in prurigo nodularis and […]
Dermavant Submits Supplemental sNDA to FDA for VTAMA Cream for AD in Adults and Children 2 Years of Age and Older
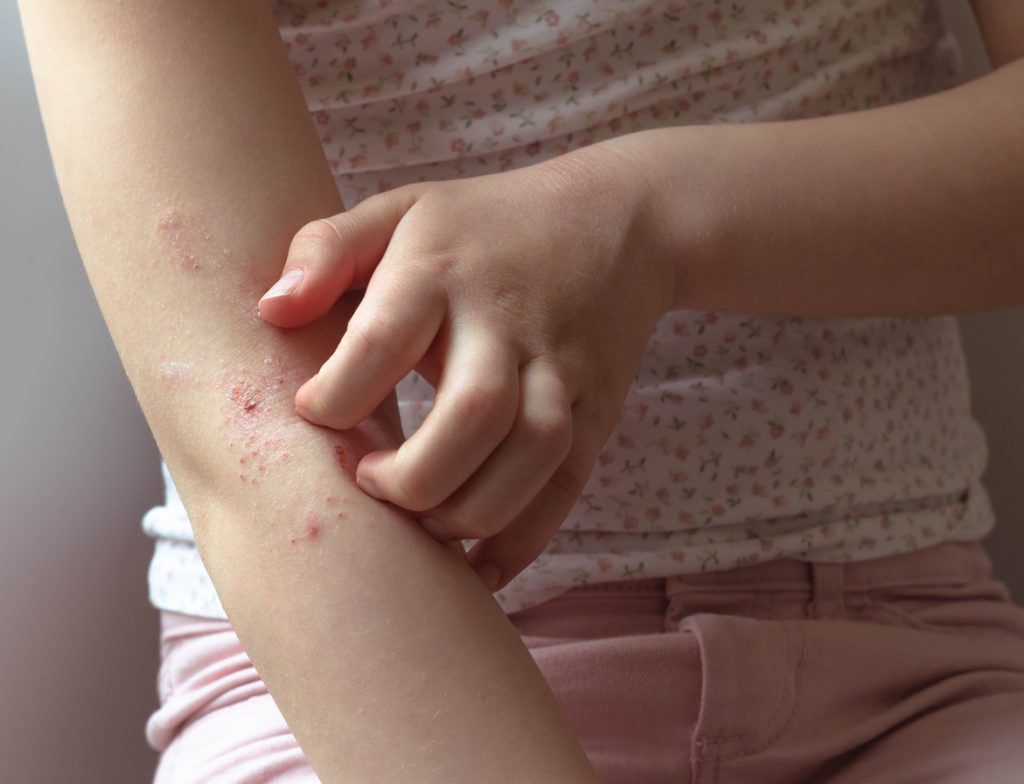
Dermavant Sciences has submitted a Supplemental New Drug Application (sNDA) to the U.S. Food and Drug Administration (FDA) for VTAMA (tapinarof) cream, 1% for the topical treatment of atopic dermatitis (AD) in adults and children 2 years of age and older. “We are confident, that VTAMA cream, if approved by the FDA, will be well […]
