FDA Fast Tracks Certa Therapeutics’ FT011 for Systemic Sclerosis

The U.S. Food and Drug Administration (FDA) has granted Fast Track Designation to Certa Therapeutics’ investigational therapy FT011 for the treatment of systemic sclerosis. FT011 is a novel, first-in-class oral therapy for the treatment of chronic fibrosis in multiple organs. It targets a GPCR receptor, GPCR68, with an extensive body of data demonstrating promising efficacy […]
IFPA Releases New Psoriatic Care Roadmap and Action Playbook for Asia

IFPA has released the Psoriatic Care Roadmap and Action Playbook for Asia. The new resources are guides featuring advocacy demands and practical strategies that aim to elevate psoriatic care across the continent. The roadmap addresses four crucial themes that emerged from the IFPA Forum Asia 2023, held in Singapore: 1. Access to Care for People with Psoriatic Disease 2. Addressing and Managing Comorbidities […]
PBUTs Linked to Itching in Hemodialysis Patients
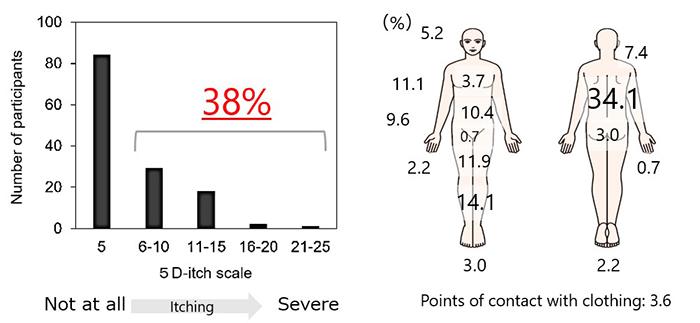
Several uremic toxins may cause itching in hemodialysis patients, new research suggests. Although its exact causes remain unclear, itching in hemodialysis patients has been associated with elevated levels of β2-microglobulin, calcium, phosphorus, or parathyroid hormone in the blood. Subsequently, improvements in hemodialysis therapy and pharmacological treatments have led to changes in the severity of itching and […]
Almirall Licenses an Anti-IL-21 Monoclonal Antibody From Novo Nordisk to Develop It as a First-in-Class Agent in Dermatology

Almirall S.A. has entered into an exclusive license agreement with Novo Nordisk for rights to the IL-21 blocking monoclonal antibody NN-8828. Under the agreement, Almirall obtains global rights to develop and commercialize this monoclonal antibody in certain fields, including immune inflammatory dermatological diseases. NN-8828 has the potential to block the activation of the downstream signaling pathways […]
