By Bob Kronemyer | Reviewed by Drs. Tyring, Schlesinger, and Lain

Founder and Director, Clinical Research Center of the Carolinas Charleston, South Carolina

STEPHEN TYRING, MD, PhD
Clinical Professor of Dermatology University of Texas Health Science Center Houston, Texas

Chief Medical Officer
Sanova Dermatology
Austin, Texas
Tirbanibulin 1% ointment (Klisyri; Almirall SA) for treating actinic keratosis of the face and scalp is now commercially available in the United States.
Results of 2 identically designed phase 3 trials of the drug, which was FDA-approved in December 2020, were recently published in The New England Journal of Medicine.1
The 2 studies totaled 702 adult patients with actinic keratoses, who were randomly assigned, in a 1:1 ratio, to receive either topical tirbanibulin or vehicle (placebo) ointment to the face or scalp (see Figure 1).
The ointment was applied by the patients to a 25 cm2 contiguous area containing 4 to 8 lesions, once daily for 5 consecutive days.
Complete clearance in trial 1 occurred in 44% of the patients in the tirbanibulin group, compared to only 5% of those in the vehicle group.
In trial 2, the percentages were 54% and 13%, respectively.
The percentage of patients with partial clearance was also significantly higher in the tirbanibulin groups: 72% vs 18% for placebo.
At 1 year, the recurrent lesion rate was 47% among patients who attained a complete response to the medication.
Mechanism of action
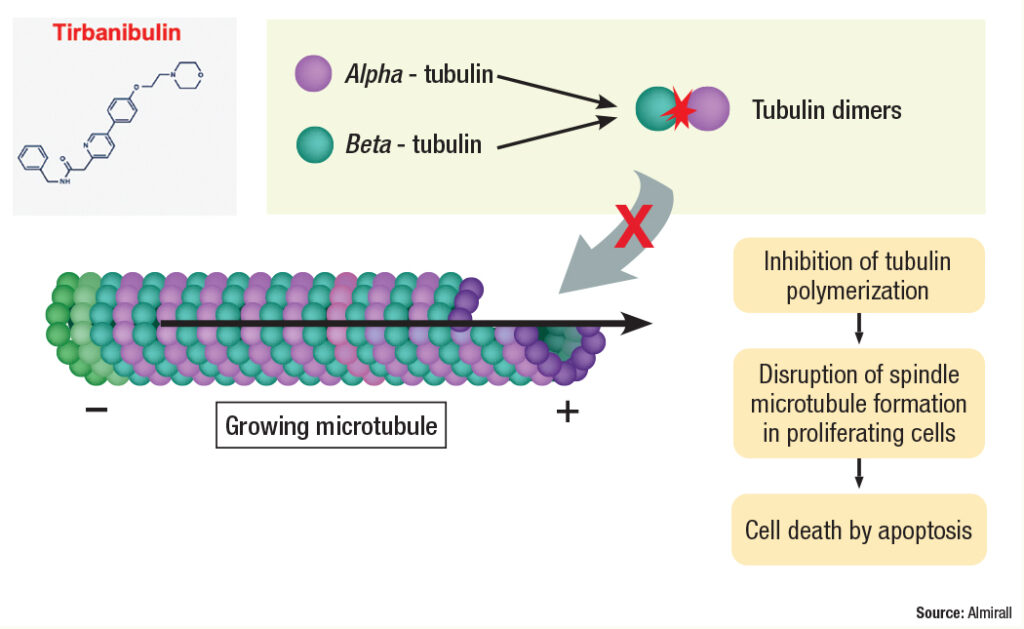
“Actinic keratosis is associated with proliferating atypical keratinocytes and p53 mutations that render them resistant to apoptosis,” said co-author Todd Schlesinger, MD, the Founder and Director of the Clinical Research Center of the Carolinas in Charleston, South Carolina. “Alpha and beta tubulin come together to form dimers, or pairs that become part of the growing microtubule.” (See Figure 2.)
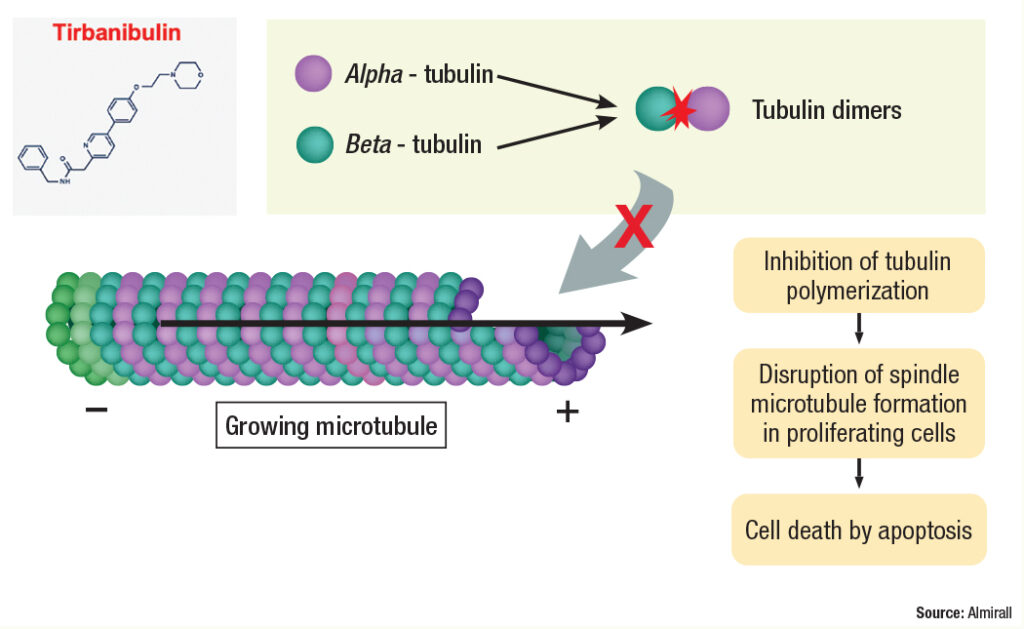
Tubulin helps form the cytoskeleton and maintain the structure of the cell, according to Dr. Schlesinger. “Tubulin functions in cell transport by allowing proteins to move along the surface of the formed microtubule,” he said. “It also plays a major role in cell division by pulling the 2 chromosomes apart during cell division, creating 2 cells each with a copy of the genetic material.”
Tirbanibulin exerts its effect by binding tubulin and preventing dimerization, thus inhibiting its polymerization. “It also inhibits Src kinase signaling, which results in increased p53 expression and the arrest of cell division at interphase Gap 2 and mitosis in fast-proliferating cells,” Dr. Schlesinger said.
Dr. Schlesinger is pleasantly surprised by the study results. “Because of the drug’s unique mechanism of action, there is little or no inflammation as opposed to necrosis, and hence there were reduced side effects compared to other treatments,” he said. “The good tolerability profile, along with a shorter treatment regimen, means greater patient acceptance and improved compliance. Research shows that compliance increases with shorter treatment durations.”
Promising results
Co-author Edward Lain, MD, Chief Medical Officer at Sanova Dermatology in Austin, Texas, noted that none of the study outcomes are unexpected to him. “During my experience as an investigator for the clinical trials, I noticed very good efficacy, as well as very good tolerability,” he said.
Recurrent actinic keratosis was strictly defined as those lesions that were present in the treatment field at baseline and appeared again by the 1-year follow-up. “The incidence was 47%, which compares favorably to data we have on other topical therapies for actinic keratosis, although no head-to-head studies are available,” Dr. Lain said. “Hence, this finding is not unexpected at all. Actinic keratosis is the symptom of the disease actinic damage; therefore, because the actinic damage persists, we will continue to see actinic keratosis arising in the treatment area.”
Dr. Lain predicts that tirbanibulin “will leap to the top” of the armamentarium for treating actinic keratosis. “Compliance should not be an issue,” he said. “Over 99% of patients completed the 5-day course of treatment. That is such a short course and it is only once a day.”
In the phase 3 trials, the peak local skin reaction (LSR) score occurred at day 8, which is 3 days after completing treatment. “Hence, LSRs should not mitigate the treatment course at all,” Dr. Lain said. “In fact, many of my patients felt they were not receiving active medication because the LSRs were so minimal, like redness, scaling and crusting, which is common with available field therapy agents.”
Despite tirbanibulin having a limited treatment area, Dr. Lain believes the medication
will be popular, due to its excellent tolerability. “A traditional therapy on the entire face, such as fluorouracil cream or imiquimod cream, has both a longer treatment course and much higher rates of LSRs,” he said.
Overall, Dr. Lain is highly excited for tirbanibulin to launch. “It will be a game changer for both dermatologists and their patients,” he said.
Co-author Stephen Tyring, MD, PhD, a Clinical Professor of Dermatology at the University of Texas Health Science Center in Houston, is also not surprised by study results, “considering both the mechanism of action of tirbani
bulin and data from previous clinical observations,” he said. “The mechanism of action of the medication is via inhibition of tubulin polymerization and Src kinase signaling.”
Dr. Tyring said tirbanibulin represents an advancement in therapy, “due both to its short duration of application and its well-tolerated adverse events. Other therapies either produce more pain, such as cryotherapy, or require longer durations of therapy, like imiquimod.”
The most common local reaction to tirbanibulin was erythema in 91% of patients, followed by flaking or scaling in 82%.
The 2 most prevalent adverse events were application pain (in 10% of patients) and pruritus, which both resolved.
Further studies are required to show efficacy and safety for areas of the skin greater than 25 cm and in combination with cryotherapy, for example, according to Dr. Tyring.
Dr. Schlesinger acknowledged that treating a smaller area is a limitation of the study, but is in line with prior studies and drug approvals. “It would be nice to have a larger area in the future,” he said. “As with current treatments for actinic keratosis, physicians may find a way to treat larger areas with the dose provided as an off-label extension.”
Investigating a larger area “would be a prudent move, especially given the favorable adverse event profile with the small field,” Dr. Schlesinger said. “Meanwhile, the small field allows us to treat the affected areas in many patients, such as the forehead, the two cheeks, or the central part of the scalp.”
Tirbanibulin might also play a role in treating nonmelanoma skin cancer. “The medication has been looked at to inhibit keratinocyte proliferation in-vitro, and even had effect in some melanoma cell lines and in a number of other tumors such as breast, stomach, prostate, bone, ovarian, and others for which it is under development,” Dr. Schlesinger said.
Dr. Schlesinger believes the dermatologic community will benefit from longer trials of tirbanibulin for actinic keratosis to further delineate the benefits and risks. “Actinic keratosis is a chronic recurring disease,” he said. “I think that dermatologists will try to combine the new medication with other currently used modalities for actinic keratosis to see if patients can achieve an even better response and reduce recurrence over time.”
DISCLOSURES
Dr. Lain has served as an investigator, consultant, speaker, and advisor to Almirall.
Dr. Tyring reports no relevant financial interests.
Dr. Schlesinger serves as an investigator, speaker and ongoing consultant for Almirall and was an investigator for Athenex (formerly Kinex), the developer of tirbanibulin. He also conducts research for other dermatologic companies such as Galderma and receives consulting and speaking fees from Sun Pharmaceutical Industries.
REFERENCE
1. Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040