Spinosad 0.9% provides new scabies option
With Christopher Belcher, MD
Amid a climate of increasing drug resistance, the April 2021 approval of Spinosad 0.9% (Natroba, ParaPRO) for scabies gives practitioners a novel prescription option with high efficacy and safety.

“This should be viewed as a very exciting development in the treatment of scabies. Given the rigorous conditions under which Spinosad was tested and the excellent safety, it’s going to be a great option for many patients who meet the indications,” said Christopher Belcher, MD, Director of Pediatric Infectious Disease at Infectious Disease of Indiana in Carmel, Indiana.
Approved in 2011 for head lice in children and adults, Spinosad 0.9% is the first new drug to earn the scabies indication in more than 30 years. Derived from fermentation of Saccharopolyspora spinosa, a naturally occurring soil actinobacterium, Spinosad works through multiple channels to cause neuronal overexcitation leading to death in multiple insect species.
In two phase 3 trials that included 551 patients total, the drug met FDA objective criteria for complete cure, which replaced investigator assessments in 2020, in patients as young as four years old.1
“Unlike previous products such as 5% permethrin, which earned approval under a subjective measure of healing, Spinosad’s approval required both complete resolution of symptoms clinically and objective resolution of all evidence of scabies by microscopy or dermatoscopy. So it was a very rigorous endpoint,” Dr. Belcher noted.
To reflect real-world conditions, investigators enrolled approximately 120 index patients with active scabies infestations and the FDA-required enrollment of all household members (up to six patients). “Scabies is a disease of close contact,” he said. “The FDA appropriately did not want to risk reinfections after treatment; therefore, entire households were required to be treated.”

Subjects performed a single hairline-to-soles application and left the treatment on for at least six hours. One month later, 78.1% of treated patients achieved complete clinical and objective cure, versus 39.6% of placebo-treated patients (P<him .0001).
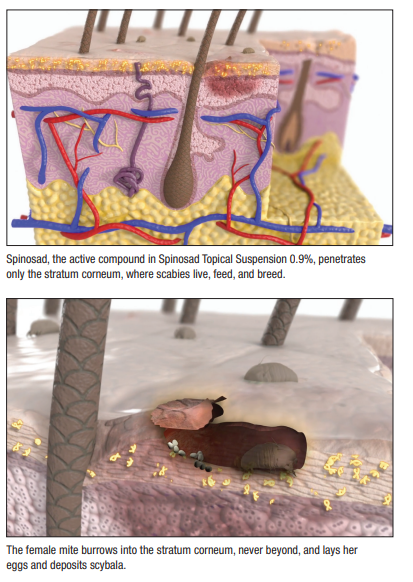
Reasons for the high placebo response rate remain unclear. The vehicle itself, which investigators used as placebo, could provide some therapeutic effect on its own, Dr. Belcher said. Whereas female mites burrow into the stratum corneum, he added, males stay on the surface and could have been mechanically removed by vehicle application, or by any manual rubbing and scratching. Still, investigators note, the difference between cure rates in the active and vehicle groups (38.4%) was both statistically significant and clinically meaningful.
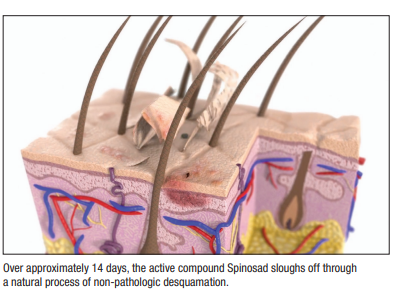
Moreover, the drug’s limited penetration enhances both efficacy and safety. “This is a unique agent because the active ingredient does not penetrate past the stratum corneum, where the parasite lives. So it’s very well-suited to treat scabies without systemic absorption, which hasn’t been shown with this agent previously.” In fact, study patients tolerated Spinosad very well—only three patients experienced adverse reactions, all of which were mild topical reactions.
Spinosad’s approval comes at a time when scabies organisms increasingly show resistance to existing therapies. “Like many other drugs we’ve seen, when you use an agent against a life form, those that are resistant tend to survive. And we’ve seen increasing resistance to permethrin or pyrethroids in many families of insects, as well as arachnids.2-4 That’s been a concern over time.” Additional prescription products approved for scabies include lindane and crotamiton.
“We see clinical failures with the other FDA-approved agents, and presumably some of these are due to resistance,” said Dr. Belcher, who treats a couple of patients with scabies monthly. Although resistance to current prescription therapies cannot be confirmed clinically, he said, dermatologists can send samples to researchers such as John Marshall Clark, PhD, Professor of Environmental Toxicology and Chemistry and Director of the Massachusetts Pesticide Analysis Laboratory at the University of Massachusetts Amherst, for that purpose.
Although reasons for the lack of resistance to Spinosad remain unknown, Dr. Belcher said, probable explanations include the novelty of its drug class and the fact that it has not been in widespread use for scabies and lice for long. Additionally, said Dr. Belcher, Spinosad has long been used environmentally for Aedes aegypti control, and no crossover resistance has emerged.
Already, the product is well-accepted in the marketplace. “There’s a lot of familiarity with it because of Natroba’s use in head lice. It’s already something that many insurers have on their formularies due to that indication. So the uptake has been very good. Between the brand name Natroba and the authorized generic Spinosad, it’s covered on more than 92% of health plans in America.”
Clinical trial data regarding Spinosad’s performance against head lice gave the product a head start in formularies with respect to scabies, he noted, and ParaPRO is working with a few remaining insurers to add the new indication. “Aside from the few formularies that are being worked on actively, I believe Spinosad is a new treatment that most physicians will find easy to prescribe.”
By John Jesitus
REFERENCES
1. Seiler JC, Keech RC, Aker JL, Miller W, Belcher C, Mettert KW. Spinosad at 0.9% in the treatment of scabies: efficacy results from 2 multicenter, randomized, double-blind, vehicle-controlled studies [published online ahead of print, 2021 Aug 12]. J Am Acad Dermatol. 2021;S0190-9622(21)02290-8. doi:10.1016/j.jaad.2021.07.074
2. Elsner E, Uhlmann T, Krause S, Hartmann R. Anstieg von skabies und therapierefraktärität bbei Bundeswehrangehörigen: acht-jahre-follow-up-studie aaus der Hautklinik des Bundeswehrkrankenhauses Berlin (2012–2019) [Increase of scabies and therapy resistance among German military personnel: an 8-year follow-up study in the Department of Dermatology of the Armed Forces Hospital Berlin, Germany (2012-2019)]. Hautarzt. 2020;71(6):447-454. doi:10.1007/s00105-020-04608-0
3. Hackenberg B, Horváth ON, Petachti M, Schult R, Yenigün N, Bannenberg P. Skabiestherapie in Deutschland: ergebnisse einer bundesweiten umfrage mit besonderem fokus auf die wirksamkeit der erstlinientherapie mit permethrin [Scabies therapy in Germany: results of a nationwide survey with a special focus on the efficacy of first-line therapy with permethrin]. Hautarzt. 2020;71(5):374-379. doi:10.1007/s00105-020-04561-y
4. Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis. 2017;11(11):e0005920. Published 2017 Nov 30. doi:10.1371/journal.pntd.0005920
DISCLOSURES
Dr. Belcher has served as a speaker, investigator, and consultant to ParaPRO.
