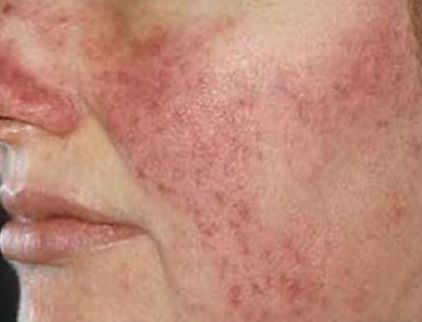
Silica microencapsulated 5% benzoyl peroxide cream (Epsolay) delivers a safe, non-irritating treatment for inflammatory (papulopustular) rosacea in adults
by Ted Rosen

The Food and Drug Administration has recently approved silica microencapsulated 5% benzoyl peroxide cream (Epsolay, Galderma) for the treatment of inflammatory (papulopustular) rosacea in adults. This product utilizes a novel technology whereby the active ingredient is incorporated within a shell composed of silica-based microcapsules. The active ingredient then slowly migrates out from the encapsulation, delivering a safe and non-irritating dose to the skin. Epsolay® approval marks the second silica-based microencapsulated agent, the first being Twyneo® (benzoyl peroxide 3% and tretinoin 0.1%), which is FDA approved for acne.
Epsolay approval was based on data derived from two parallel, double-blind, vehicle-controlled phase 3 trials (NCT 03448939 and 03564119), which evaluated the safety and efficacy of the drug in 933 subjects with moderate to severe papulopustular rosacea. Patients were randomly selected to receive either active or vehicle (placebo) once daily for twelve weeks. The co-primary endpoints were Investigator Global Assessment (IGA) score of clear or almost clear at the end of 12 weeks and the absolute reduction compared to baseline in inflammatory lesion count.
Trial results disclosed that 47.4% and 49.2% of patients in the two trials reported IGA treatment success while using microencapsulated benzoyl peroxide compared to 20.7% and 28.2% of patients treated with vehicle. At trial end, inflammatory lesion count was reduced by 68.2% and 69.4% in those who received the active drug, compared to 38.3% and 46.0% among those receiving vehicle. A significantly greater treatment effect for encapsulated benzoyl peroxide relative to vehicle was noted as early as week 2 of the trials. The pivotal trials were followed by an open-label, one-year extension trial in which 73% of Epsolay®-treated patients achieved IGA success at week 52.
The most common side effects reported during the trials were application site reactions, such as erythema (2%), pain (2%), edema (1%), and pruritus (1%). Not only does this provide another therapeutic option for rosacea, but it also further demonstrates the utility of this exciting novel drug formulation technology.
Reference:
“A Study of S5G4T-1 in the Treatment of Papulopustular Rosacea.” NIH U.S. National Library of Medicine. Available at: https://clinicaltrials.gov/ct2/show/results/NCT03448939 and https://clinicaltrials.gov/ct2/show/results/NCT03564119 Accessed: May 2, 2022.