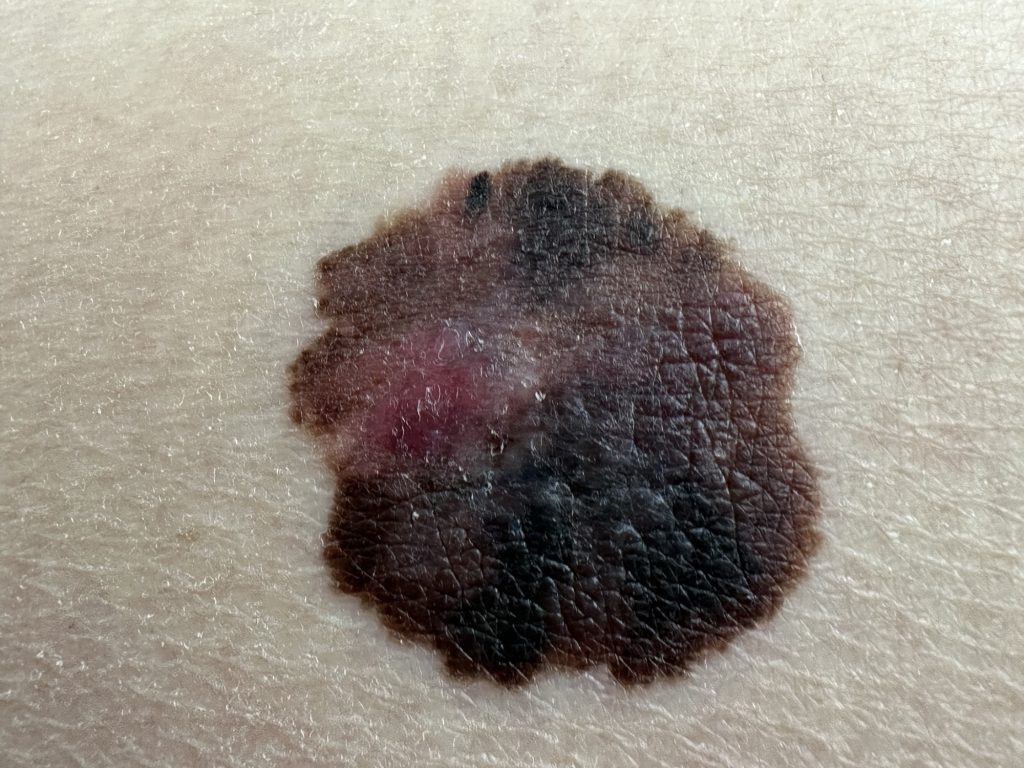
Scientists have made significant progress in understanding the molecular mechanisms leading to melanoma development, but precisely how the PTEN protein regulated melanoma progression was unclear until now.
In a new study published in Cancer Research, Moffitt Cancer Center researchers showed how the antitumor activity of PTEN suppresses the cancer-promoting activity of the FRA1 transcription factor through the AKT signaling pathway.
Mutations in PTEN commonly occur during melanoma progression, blocking its tumor-inhibitory activity and causing tumor cells to develop more aggressive characteristics. PTEN serves several functions, including inhibition of the AKT signaling pathway, which promotes tumor progression by stimulating processes such as proliferation, survival and invasion.
Numerous studies in melanoma and other cancers have shown that reduced activity of PTEN enhances signaling through the cancer promoting AKT pathway. However, AKT inhibitors have not been effective in treating patients with melanoma in clinical trials, suggesting that scientists still do not entirely understand the molecular mechanisms of PTEN tumor suppression and AKT tumor promotion in melanoma.
“The underwhelming preclinical and clinical performances of these inhibitors have prompted two fundamental questions regarding PTEN-mediated melanoma suppression,” says Florian Karreth, Ph.D., associate member of the Department of Molecular Oncology at Moffitt in Tampa, FLA., in a news release. “First, does PTEN suppress melanoma through its lipid phosphatase activity? And second, is AKT a crucial signaling node in PTEN-deficient melanoma?”
Dr. Karreth and a team of Moffitt researchers performed cell culture and mouse model studies to determine how PTEN inhibits melanoma development and whether alterations in PTEN lead to AKT tumor promotion activity. They found that PTEN predominantly inhibits melanoma cell proliferation, invasion and tumor growth through its lipid phosphatase activity. By contrast, its protein phosphatase and scaffolding activity have limited to no inhibitory effects on melanoma progression.
Additional investigations found that AKT activity was able to overcome the antitumor activity of PTEN to promote melanoma growth and invasion, suggesting that the regulation of AKT by PTEN is particularly important at the later stages of melanoma formation such as metastasis. The researchers also determined that AKT controlled the production of the FRA1 protein via the mTOR regulator of protein translation to promote melanoma progression.
“Taken together, not only does the discovery of the AKT/mTOR/FRA1 axis add to our understanding of AKT-mediated melanomagenesis, but it may also offer new opportunities for targeting PTEN-deficient melanoma,” Dr. Karreth says.