With Todd Schlesinger, MD
Pembrolizumab extension provides preferred option

The July approval of pembrolizumab (Keytruda, Merck) for locally advanced cutaneous squamous cell carcinoma (laCSCC) not curable by surgery or radiation is helping to raise the standard of care for patients who previously had scant alternatives.
“Where there was previously no standardized management approach to these advanced tumors,” said Todd Schlesinger, MD, “we now have two approved therapies. The National Comprehensive Cancer Network (NCCN) has given programmed death ligand 1 (PD-L1) inhibitors a category 2A preferred recommendation over previously used therapies.1 Dermatologists treating advanced skin cancer now have options that they didn’t before—with better outcomes than before.”
Before the approval of cemiplimab (Libtayo, Regeneron Pharmaceuticals, and Sanofi Genzyme) for laCSCC and metastatic CSCC (mCSCC) not amenable to curative surgery or radiation, physicians treated advanced CSCC with various off-label therapies, including epidermal growth factor receptor inhibitors such as cetuximab, and/or platinum-based chemotherapy, radiation therapy, and other biologics, all with limited results. Pembrolizumab adds another effective therapy, with an acceptable safety profile, for laCSCC and recurrent or metastatic CSCC (r/mCSCC).
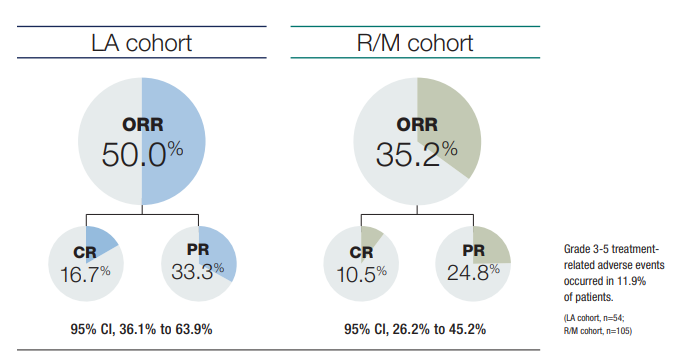
In the second interim analysis (IA) of the KEYNOTE-629 phase 2 study, the 54-patient laCSCC cohort achieved an objective response rate (ORR) of 50% (95% confidence interval/CI 36.1% to 63.9%).2 At a median follow-up longer than 1 year (median time from the first dose to data cutoff 14.9 months), 9 patients (16.7%) achieved complete responses, and 18 (33.3%) achieved partial responses. The median time to response was 2.6 months, with initial responses observed by week 6 in 14 of 27 confirmed responders. The median duration of response was not yet reached; 88.1% and 84.1% of responses lasted at least 6 months and at least 12 months, respectively.
The foregoing data add longer-term follow-up, both in patients who had (22.2%) and had not undergone previous systemic therapies than was previously available. In the first KEYNOTE-629 IA (median follow-up 11.4 months), 105 patients with r/mCSCC achieved an ORR of 34.3%, including 4 complete responses and 32 partial responses.3
The longer-term data moreover begin to reveal stratification. The laCSCC group experienced a higher ORR than did the r/mCSCC cohort (35.2%). Investigators noted, however, that since the initial KEYNOTE-629 analysis, 7 more patients in the r/mCSCC cohort advanced from partial to complete responses, suggesting that in responders, the benefit of checkpoint inhibition can increase over time.
Although responses occurred regardless of PD-L1 expression levels, Dr. Schlesinger added, investigators observed a trend toward improved ORR with increasing PD-L1 levels. Somewhat similarly, a French trial involving 57 systemic treatment-naïve patients with unresectable CSCC (CARSKIN) who received first-line pembrolizumab monotherapy showed a significantly higher ORR for PD-L1-positive patients than PD-L1-negative patients (55% versus 17%; P = .02) at a median follow-up of 22 months.4 In the first KEYNOTE-629 analysis, ORR for PD-L1-positive versus negative patients was 33.3% versus 20%.3
Also consistent with the first IA, subgroup analysis of the r/mCSCC cohort in the second IA revealed more robust and potentially faster responses in patients who received pembrolizumab as first-line therapy versus later (ORR 50% versus 33%, respectively).
Additionally, high tumor mutational burden (TMB) has been shown to predict immunotherapy efficacy, including that of pembrolizumab.5,6 Because UV-induced DNA damage creates high TMB in nonmelanoma skin cancers including SCC, CSCC is believed to be susceptible to immunotherapy. Conversely, CARSKIN authors note that because CSCC in immunocompromised patients (who were excluded from this study) carries lower TMB, such patients may respond poorly to anti-PD-L1 therapies.
Due to limited data, the clinical utility of the above observations remains uncertain. “However, such distinctions may assume importance—differences in response rates according to patient characteristics such as PD-L1 status or prior checkpoint-inhibitor exposure may help guide clinical decision-making when more data become available,” said Dr. Schlesinger. The responsiveness of laCSCC to anti-PD-L1 agents also suggests potential benefit in the adjuvant setting following surgery and radiation therapy, scenarios that are under investigation.
Safety data in the most recent analysis are consistent with pembrolizumab’s established safety profile, and that of cemiplimab. Although patients in the second IA were otherwise relatively healthy, the median age in the laCSCC and r/mCSCC cohorts was 75.5 and 72 years, respectively. Altogether, 69.2% of patients experienced at least 1 treatment-related adverse event (AE)—most commonly pruritus (18.2%), fatigue (14.5%), and asthenia (12.6%). However, most AEs were mild-to-moderate, and only 14 patients (8.8%) discontinued due to treatment-related AEs. Similarly, 22.6% of patients experienced immune-mediated AEs or infusion reactions, but only 8.2% of these AEs were grade 3 to 5. To minimize patient morbidity and mortality, early recognition and management of AEs—especially immune-mediated and endocrine-related AEs—is critical.
“With a median follow-up of more than one year in the laCSCC cohort and approximately two years in the r/mCSCC cohort,” Dr. Schlesinger noted, “the new data confirm the rapid, robust activity of pembrolizumab against cSCC. The treatment also shows durable efficacy and promising survival with manageable safety.”
By John Jesitus
REFERENCES
1. Schmults CD, Blitzblau R, Aasi SZ, et al. National Comprehensive Cancer Network guidelines version 2.20 21: squamous cell skin cancer. https://www.nccn.org/guidelines/category_1. Published August 16, 2021. Accessed October 13, 2021.
2. Hughes BGM, Munoz-Couselo E, Mortier L, et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): an open-label, nonrandomized, multicenter, phase II trial. Ann Oncol. 2021;32(10):1276-1285. doi:10.1016/j.annonc.2021.07.008
3. Grob JJ, Gonzalez R, Basset-Seguin N, et al. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: a single-arm phase II trial (KEYNOTE-629). J Clin Oncol. 2020;38(25):2916-2925. doi:10.1200/JCO.19.03054
4. Maubec E, Boubaya M, Petrow P, et al. Phase II study of pembrolizumab as first-line, single-drug therapy for patients with unresectable cutaneous squamous cell carcinomas. J Clin Oncol. 2020;38(26):3051-3061. doi:10.1200/JCO.19.03357
5. Ott PA, Bang YJ, Piha-Paul SA, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37(4):318-327. doi:10.1200/JCO.2018.78.2276
6. Yarchoan M, Albacker LA, Hopkins AC, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight. 2019;4(6):e126908. Published 2019 Mar 21. doi:10.1172/jci.insight.126908
DISCLOSURES
Dr. Schlesinger is a consultant, investigator, and speaker for Regeneron and a consultant for Genentech.