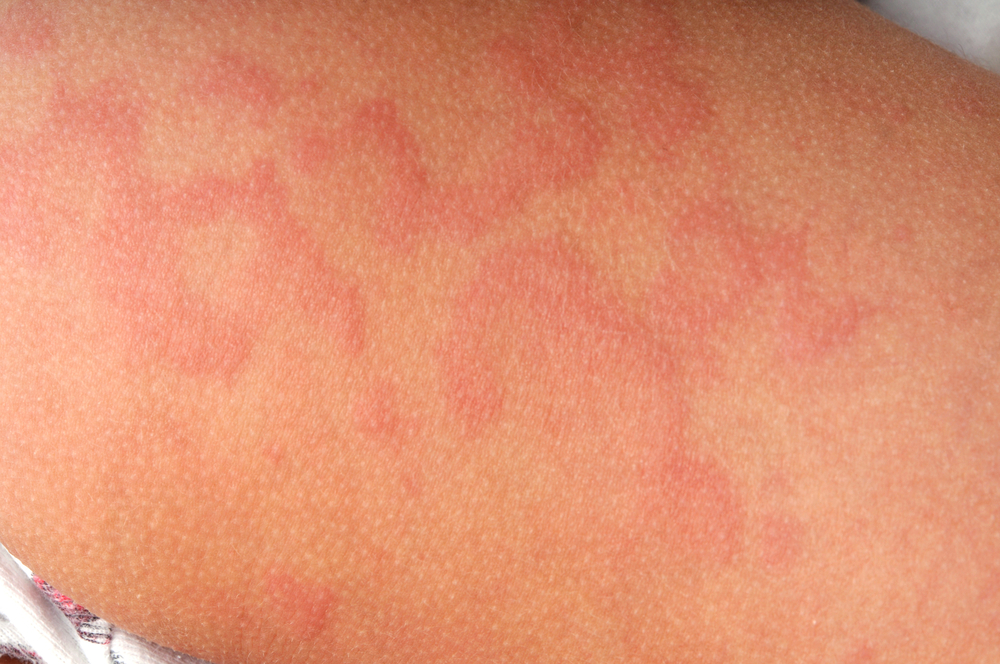
The Ministry of Health, Labor and Welfare (MHLW) in Japan has granted marketing and manufacturing authorization for dupilumab (Dupixent, Sanofi and Regeneron Pharmaceuticals, Inc.) for the treatment of chronic spontaneous urticaria (CSU) in people aged 12 years and older whose disease is not adequately controlled with existing therapy.
Japan is the first country to approve dupilumab for CSU.
The Japanese approval is based primarily on data from Study A of the LIBERTY-CUPID clinical trial program evaluating dupilumab as an add-on therapy to standard-of-care H1 antihistamines compared to antihistamines alone (placebo) in 138 patients with CSU who remained symptomatic despite antihistamine use and were not previously treated withomalizumab (Xolair, Genentech USA, Inc., and Novartis Pharmaceuticals). This study met the primary and all key secondary endpoints. Patients taking dupilumab added to standard-of-care antihistamines experienced a significant reduction in itch severity compared to standard of care alone at 24 weeks. The safety profile of dupilumab in CSU was generally consistent with the known safety of Dupixent in its approved dermatological indications.
In addition to CSU, dupilumab is approved in Japan in certain patients with atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis (CRSwNP), and prurigo nodularis.
The potential use of dupilumab in CSU is under clinical development in additional countries around the world, and its safety and efficacy have not been fully evaluated by any regulatory authority outside of Japan.