TDD Industry News and Views: Weekly Updates on Comings, Goings, and Other Happenings in Dermatology

Sudo Biosciences Raises $116M Series B Financing to Advance TYK2 Therapeutics Programs Into the Clinic Sudo Biosciences raised $116 million Series B financing to advance two investigational TYK2 candidates into the clinic in 2024. Sudo is developing a potential first-in-class topical TYK2 inhibitor for psoriasis and other immune-mediated dermatologic diseases. In addition, Sudo’s CNS program is […]
Long-term Use of Steroid Creams Linked to Osteoporosis, Fractures

Higher doses of topical corticosteroids may increase risk of the brittle bone disease osteoporosis and associated fractures, according to research in the Journal of the European Academy of Dermatology and Venereology. Investigators selected 129,682 osteoporosis cases and 34,999 major osteoporotic fracture (MOF) cases and matched them with 518,728 and 139,996 controls (without osteoporosis or MOF) by […]
FDA Approves FILSUVEZ Topical Gel for Junctional and Dystrophic EB

The U.S. Food and Drug Administration (FDA) approved FILSUVEZ (birch triterpenes) topical gel for the treatment of partial thickness wounds in patients 6 months and older with Junctional Epidermolysis Bullosa (JEB) and Dystrophic Epidermolysis Bullosa (DEB). Chiesi Global Rare Diseases’ FILSUVEZ is the first approved treatment for wounds associated with JEB, a rare, moderate-to-severe form of […]
Laser Hair Removal Helps Reduce Recurrence of Pilonidal Disease in Teens
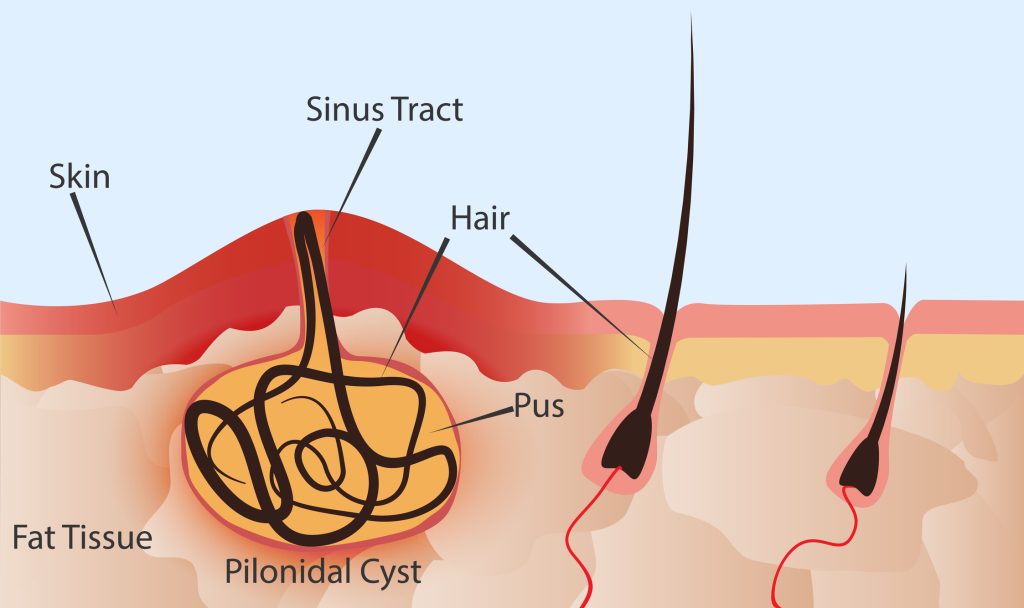
Pilonidal disease is a painful inflammatory disease that occurs when cysts form between the buttocks. These cysts often recur following standard treatment with razors or creams. Peter C. Minneci, MD, Chair of Surgery at Nemours Children’s Health in Delaware Valley, recently conducted a study1 that looked at the use of laser hair removal in these patients. He […]
Proof of Concept: Oral Heat Shock Protein 90 Inhibitor Shows Promise in HS
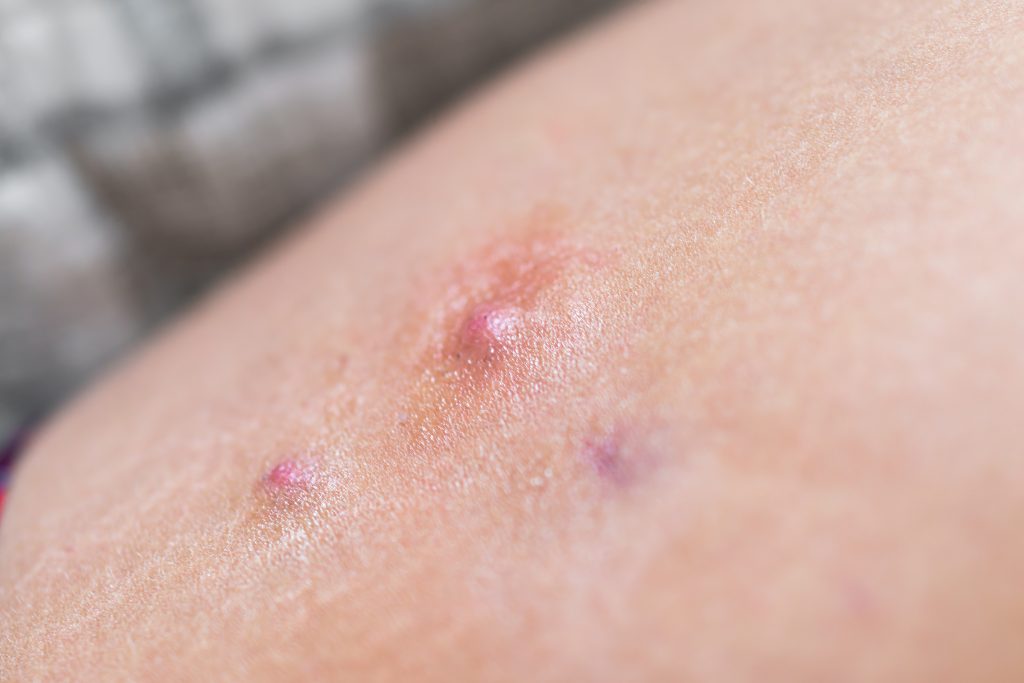
Heat shock protein 90 inhibition by oral RGRN-305 may represent a novel way of treating hidradenitis suppurativa (HS), according to a study in JAMA Dermatology. In the parallel-design double-blind trial of 15 HS patients, those patients who received oral RGRN-305, 250 mg, once daily showed a robust treatment response compared with their counterparts who received placebo after […]
TDD AD Pipeline Watch: Aldeyra’s ADX‑629 Performs Well in Phase 2 Study
Aldeyra Therapeutics’ investigational RASP modulator ADX-629 performed well in a Phase 2 clinical trial of patients with atopic dermatitis (AD), according to topline results. Aldeyra expects to initiate a multicenter, randomized, placebo-controlled Phase 1/2 clinical trial of ADX‑246 in healthy volunteers and patients with AD in the first half of 2024. Topline results from the trial […]
The American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters Release New AD Guidelines
New practice parameters from the Joint Task Force for Practice Parameter) recommend the use of topical corticosteroids or topical calcineurin inhibitors in patients with uncontrolled atopic dermatitis (AD) despite moisturizer use. The Joint Task Force is a partnership between the American College of Allergy, Asthma, and Immunology (ACAAI) and the American Academy of Allergy, Asthma […]
Study: Air Pollution Triggers Summer AD Surge
Air pollution may contribute to the development or worsening of skin conditions such as atopic dermatitis and eczema, according to researchers out of Massachusetts General Hospital (MGH). The work, which is published in Dermatology and Therapy, points to the need to improve air quality to lower the burden of skin disease, especially for vulnerable communities. For the […]
FDA OKs Can-Fite’s Plan to Study Piclidenoson in Kids With Psoriasis
The U.S. Food and Drug Administration (FDA) has green lighted Can-Fite’s plan to include children with psoriasis in studies of Piclidenoson, a small molecule, adenosine A3 receptor (A3AR) agonist. The Pediatric Study Plan would allow enrollment of children with psoriasis to Can-Fite’s upcoming Phase 3 pivotal clinical psoriasis studies, aiming at registration of Piclidenoson with both the FDA […]
Review Study Aims to Refine Safety Profile of JAK Inhibitors in AD
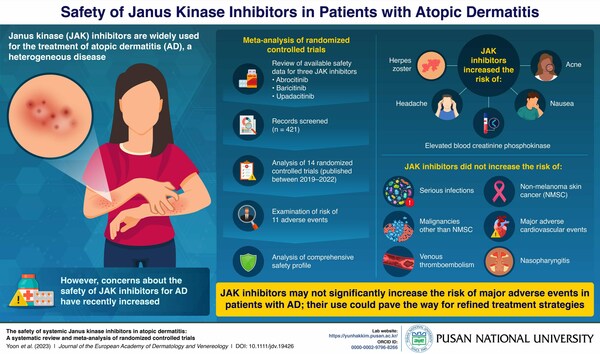
Contrary to concerns regarding malignancy, venous thromboembolism, and major adverse cardiac events, the use of Janus kinase (JAK) inhibitors is not associated with a significant increase in the overall risk for these conditions in patients with atopic dermatitis (AD), according to a systematic review and meta-analysis, published in the Journal of the European Academy of Dermatology & Venereology. Recently, […]
