FDA Approves Arcutis’ Roflumilast Topical Foam, 0.3% for Seb Derm in Individuals Aged 9 and Older

The U.S. Food and Drug Administration (FDA) has given its nod to topical roflumilast foam (Zoryve, Arcutis) for seborrheic dermatitis. The PDE4 inhibitor is the first topical treatment for seborrheic dermatitis with a new mechanism of action in more than 20 years. Arcutis aims to make the foam widely available via wholesaler and dermatology pharmacy channels […]
Research Seeks to Understand How Climate Change Affects AD, Other Allergic Conditions
Global warming and other environmental changes may be contributing to rising rates of epithelial barrier dysfunction and atopic dermatitis (AD). This is among the main messages from research published in a themed issue of Annals of Allergy, Asthma & Immunology. When the epithelial barrier is disrupted, it facilitates the entry of the external exposome into and […]
FDA Green Lights Leo’s Adbry for Kids With AD
The U.S. Food and Drug Administration (FDA) has expanded the approval of tralokinumab-ldrm (Adbry, LEO Pharma Inc) to include kids aged 12-17 years with moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Adbry is the first and only FDA-approved biologic that specifically […]
Wound Healing Breakthrough: A New Sustainable Hydrogel Based on Seaweed Exhibits Lower Skin Adhesion, Swelling

A novel, low-cost hydrogel using a component found in seaweed may aid wound healing, according to new research in the International Journal of Biological Macromolecules. The hydrogel was made using alginate, calcium carbonate, and carbonated water. Alginate is a biocompatible substance that can be extracted from beach-cast seaweed that does not adhere strongly to cells or skin tissues. […]
Almirall, Etherna Partner to Develop mRNA-based Therapies in Medical Dermatology

Almirall, S.A. (ALM) and etherna are teaming up to discover and develop new mRNA-based therapies for severe skin diseases, including non-melanoma skin cancer. The alliance combines and leverages etherna’s proprietary mRNA capabilities and lipid nanoparticle (LNP) formulations with Almirall’s medical dermatology expertise to accelerate discovery of novel treatment options. Etherna and Almirall will work collaboratively on the research […]
Better Together: Tapinarof Cream, 1% Plus Biologics Show Promise in Psoriasis
More than half of adult psoriasis patients on biologics hit the National Psoriasis Foundation treat-to-target goal of 1% body surface area (BSA) or less involvement within three months of adding Tapinarof cream, 1% (Vtama, Dermavant).
Meet the 2023 NEA Grant Winners
The National Eczema Association (NEA) announced the recipients for its 2023 research grants. This is the 20th anniversary of NEA’s grant program. So far, the NEA has invested nearly $4 million since its first grant was awarded in 2004. The 2023 research grantees include: The Champion Research Grant encourages researchers to continue research on emerging or ongoing challenges in eczema […]
Diagnose this Zebra
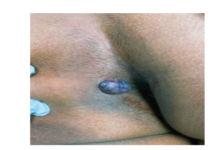
Exophytic perianal nodule With Anthony W. Linfante, MD, and Brent Kelly, MD CASE HISTORY A 52-year-old male Pacific Islander presented to the dermatology consult service with a 2-year history of a very slowly growing pigmented, exophytic perianal mass of about 2cm in diameter (Figure 1). The lesion was neither painful nor pruritic but did occasionally […]
Pediatric nonmelanoma skin cancer

With Jennifer Huang, MD Children without risk factors require vigilance “The general thinking in our specialty is that children who are otherwise healthy don’t get nonmelanoma skin cancers (NMSCs),” said Jennifer Huang, MD. “However, a study my colleagues and I published in the May issue of the Journal of the American Academy of Dermatology suggests […]
