Breaking News from Maui Derm 2024: Nemolizumab Improves Lesions, Relieves Itch in AD

Nemolizumab improves skin lesions, itch, and sleep disturbance in patients with moderate-to-severe atopic dermatitis (AD), according to results from two phase 3 studies. Galderma’s nemolizumab is a first-in-class interleukin-31 receptor alpha antagonist that is under investigation for atopic dermatitis. The research was presented by Jonathan I Silverberg, MD, Associate Professor of Dermatology and Director of Clinical […]
AA Pipeline Watch: Sun Pharma’s Deuruxolitinib Performs Well in New Phase 3 Data
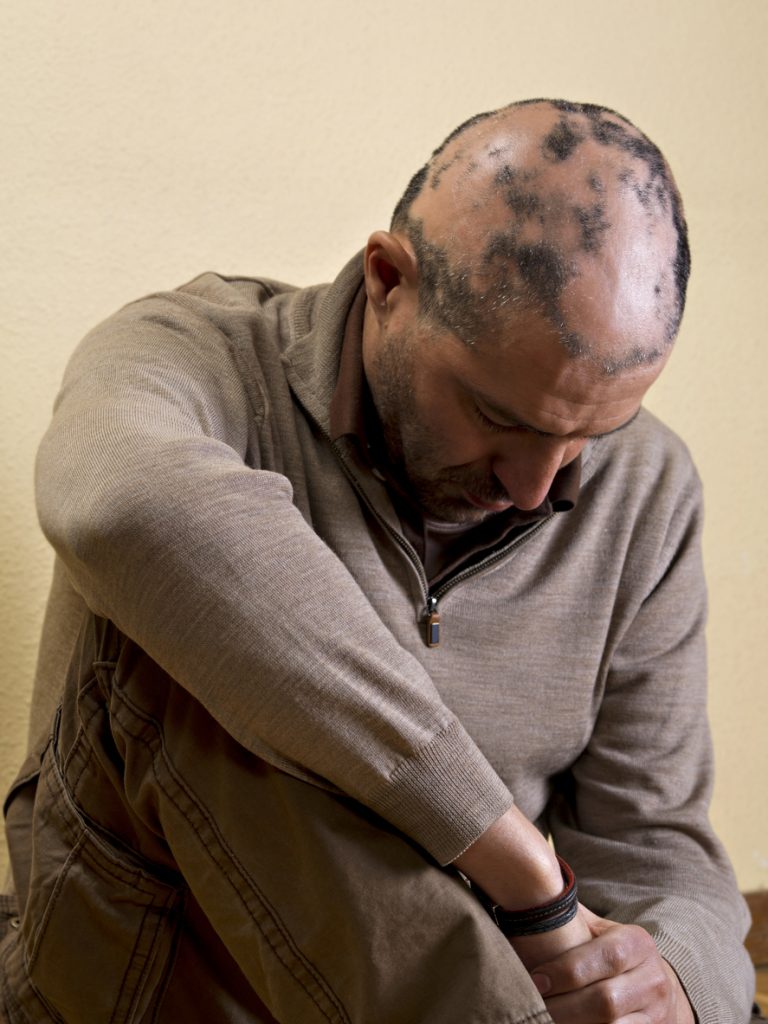
Twice-daily dosing of deuruxolitinib resulted in significant hair regrowth for adults with moderate-to-severe alopecia areata (AA), and these improvements were visible as early as 12 weeks and lasted throughout the 24-week study, according to the Phase 3 THRIVE-AA2 trial. The new results, presented as a late breaker at Maui Derm 2024, are consistent with earlier data on the Janus kinase […]
Breaking News from Maui Derm 2024: Bimekizumab Shows Maintenance of Response in HS
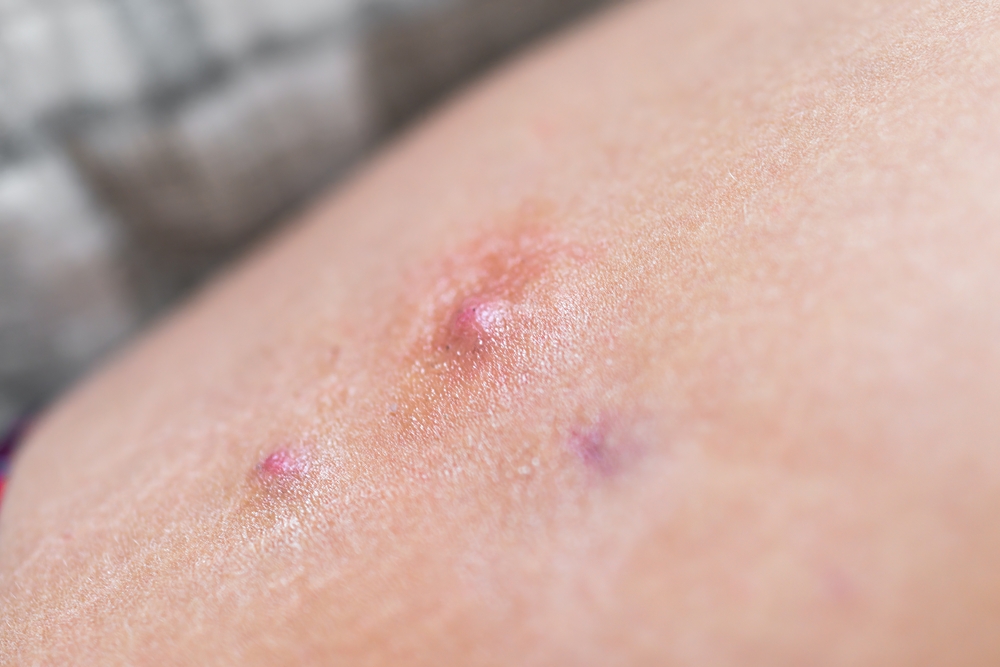
Bimekizumab demonstrated sustained response in hidradenitis suppurativa (HS), according to a late breaker presented at Maui Derm 2024. Almost all patients who responded after an initial 16 weeks of bimekizumab treatment maintained HiSCR50 (Hidradenitis Suppurativa Clinical Response) and AN count of 0, 1, or 2 clinical response rates through Wk48, the study showed. Bimekizumab, a […]
Done Deal: LEO Pharma Finalizes Acquisition of Key Assets From Timber Pharmaceuticals

LEO Pharma now owns TMB-001 as well as certain other assets from Timber Pharmaceuticals following its chapter 11 bankruptcy filing. The TMB-001 project aims to develop a topical treatment for multiple moderate to severe subtypes of congenital ichthyosis – a skin disease with significant unmet need and no approved prescription therapies available. The TMB-001 project […]
Study: Melanoma Overdiagnosis Is on the Rise in White People
More than half of all melanoma diagnoses among white Americans may be overdiagnoses, according to a new study in BMJ Evidence-Based Medicine. For the study, researchers collected incidence and mortality data from the Surveillance, Epidemiology and End Results 9 registries database. They also used DevCan software to calculate the cumulative lifetime risk of being diagnosed with melanoma between […]
Arcutis’ Zoryve Foam Is Now Available for Seb Derm Treatment in the U.S.

Arcutis Biotherapeutics, Inc.’s ZORYVE topical foam, 0.3%, is now officially available for the treatment of seborrheic dermatitis. The U.S. Food and Drug Administration (FDA) approved ZORYVE foam for the treatment of seborrheic dermatitis in individuals 9 years of age and older on December 15, 2023. Roflumilast foam (Zoryve, Arcutis) will be available via wholesaler and pharmacy […]
Health Canada Approves Galderma’s Restylane SHAYPE for Chin Augmentation
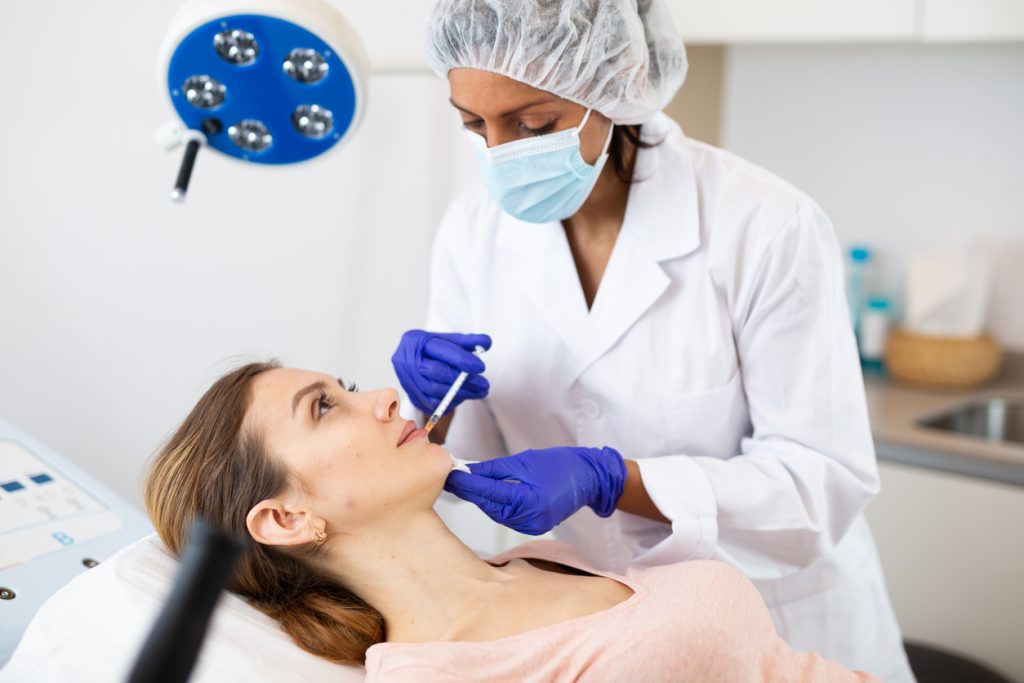
Health Canada has given its nod to Restylane SHAYPE, a hyaluronic acid (HA) injectable designed for temporary augmentation of the chin region. Galderma Restylane SHAYPE will be available in Canada as of February 2024. Other regulatory reviews around the world are ongoing, the Company reports. Powered by new NASHA HD technology from Galderma, Restylane SHAYPE has the highest G’ (“G […]
Caught Red-Handed: Exposing the Secret Life of CD4+ T Cells as Melanoma Warriors
In addition to their role in activating other immune cells, CD4+ T cells may help control melanoma, according to new research in Science Immunology. Researchers used microscopic live imaging to visualize the activities and interactions of CD4+ T cells with other cell types in the tumor microenvironment. “Our in-depth study, using animal models, unraveled the complex biology of CD4+ […]
Breaking News from Maui Derm: Research Supports the Safety and Efficacy of Delgocitinib in CHE
Delgocitinib cream continues to perform well in chronic hand eczema (CHE), with limited systemic exposure, according to two studies presented at the 2024 Maui Dermatology conference. Leo Pharma’s Delgocitinib is an investigational topical pan–Janus kinase inhibitor that inhibits activation of the JAK-STAT pathway. In one study, researchers reported significant improvements in both clinician-assessed (Investigator Global Assessment or […]
Eye on Itch: Evommune Initiaties Phase I Study of EVO756 in CSU
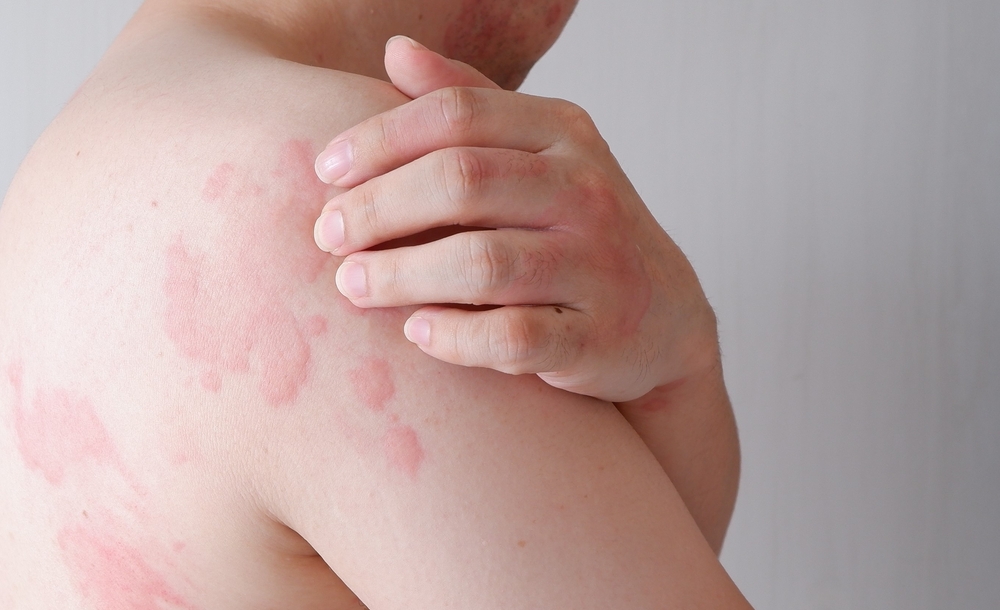
Evommune, Inc. is initiating its Phase 1 first-in-human study, evaluating EVO756 in chronic spontaneous urticaria (CSU). By blocking MRGPRX2 activation and degranulation of mast cells, EVO756 has the potential to be a first-in-class oral treatment for a variety of mast cell-mediated diseases, including chronic spontaneous urticaria and inflammatory itch. The Phase 1 study is a […]
