TDD’s Guide to Maui Derm Caribbean 2024; Stay Tuned for Daily Coverage

Approaching its second year, the Maui Derm Caribbean meeting is slated to take place at the Hilton Aruba from January 10–13, 2024. Led by Louisville, KY dermatologist Joe Fowler, MD, and George Martin, MD, a dermatologist in Kihei, HI, and Maui Derm Program Chairman, the four-day meeting will feature treatment updates on psoriasis, atopic dermatitis, acne/rosacea, pigmentation […]
ChatGPT Can Provide Reliable Information on AD

Large language models like Chat-Generative Pre-Trained Transformer (ChatGPT) have become novel resources for patients, especially as physicians struggle to keep up with the unsustainable number of electronic messages and questions they get from their patients. It turns out that Chat-GPT may be a reliable resource and supplement to physician-dispensed advice on atopic dermatitis (AD), according […]
IPC Adds to Board of Directors

André Vicente Esteves de Carvalho, MD, PhD, and Amy Paller, MS, MD, are the newest members of the International Psoriasis Council’s (IPC) volunteer Board of Directors. Dr. Carvalho is responsible for the Psoriasis Ambulatory and a researcher at the Hospital Moinhos de Vento Complexo in Porto Alegre, Brazil. Dr. Paller is the Walter J. Hamlin Professor […]
And Then There Were Two: FDA Approves Zelsuvmi for Molluscum Contagiosum
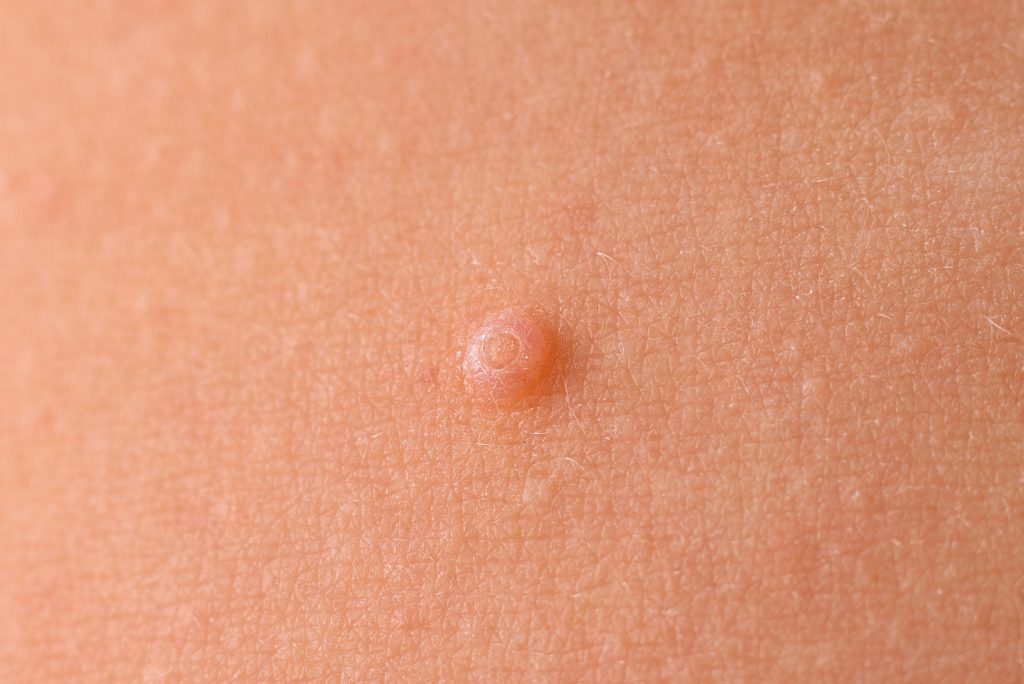
The U.S. Food and Drug Administration (FDA) has approved Zelsuvmi (berdazimer topical gel, 10.3%) for the treatment of molluscum contagiosum in adults and children one year of age and older. Zelsuvmi is expected to be commercially available during the second half of 2024, according to Ligand Pharmaceuticals. Zelsuvmi is the first novel drug for the treatment of molluscum infections […]
Last Patient Dosed in Part 2 of Verrica’s Phase 2 Study of VP-315 in BCC

The last patient has been dosed in Part 2 of Verrica Pharmaceuticals Inc.’s Phase 2 trial of VP-315, a potential first-in-class oncolytic peptide, for the treatment of basal cell carcinoma. VP-315 is a chemotherapeutic administered intratumorally. It works by inducing lysis of intracellular organelles of tumor cells such as mitochondria, thereby unleashing a broad spectrum of […]
Rosacea Pipeline Watch: Journey Medical Submits NDA for DFD-29
Journey Medical Corporation has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) seeking approval for DFD-29 (Minocycline Hydrochloride Modified Release Capsules, 40 mg) for the treatment of inflammatory lesions and erythema of rosacea in adults. DFD-29 is being developed in collaboration with Dr. Reddy’s Laboratories Ltd. “Based on the […]
Vaping Linked to AD, PsO, Burns, and More
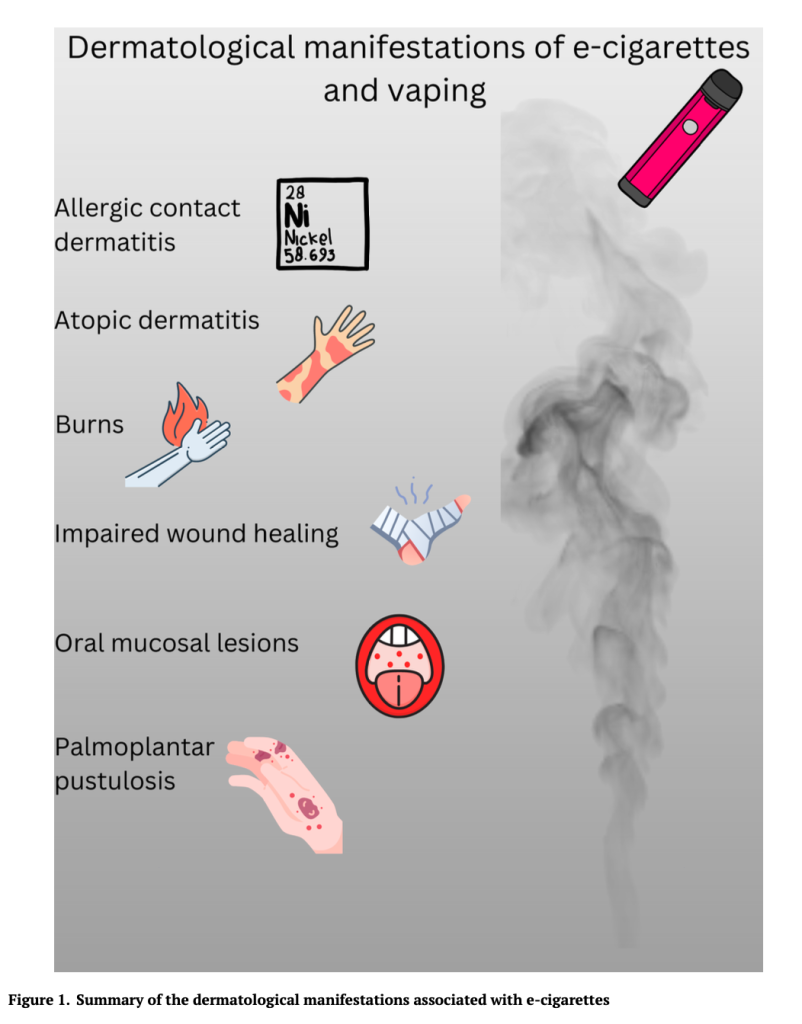
Tremendous attention has been paid to the detrimental effects of e-cigarettes on lung tissue, but less is known about how e-cigarette use affects the skin. To find out more, researchers culled the literature to better define the dermatological burden of e-cigarettes and vaping. Here’s what they found: Vaping and AD: What’s the link? Smoking is […]
Biosimilar Watch: FDA Accepts BLA for Proposed Stelara Biosimilar
The U.S. Food and Drug Administration (FDA) has accepted Accord BioPharma, Inc’s Biologics License Application (BLA) for DMB-3115, a proposed biosimilar to ustekinumab (Stelara, Johnson & Johnson). Ustekinumab is approved for the treatment of plaque psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. The BLA submission for DMB-3115 is based on results from phase III multi-regional clinical trials in […]
FDA Clears Enspectra’s VIO System for Biopsy-free Skin Imaging
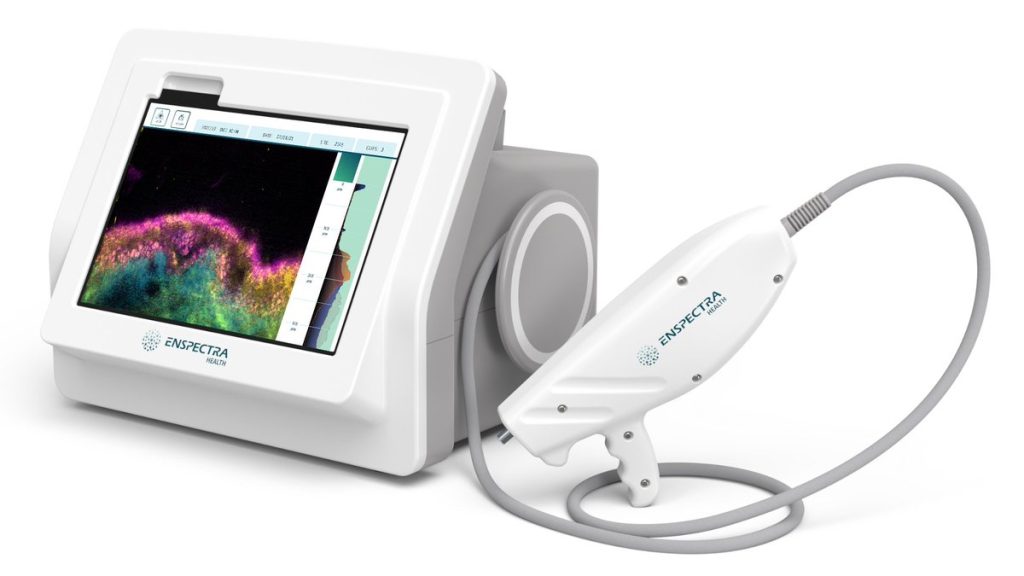
The U.S Food and Drug Administration (FDA) has granted 510(k) marketing clearance to Enspectra Health’s VIO System, a first-of-its-kind technology that combines reflectance confocal and multiphoton microscopy to enable cellular resolution, and cross-sectional imaging of skin in seconds. Vio is portable, handheld, and digitizes pathology directly from a patient’s skin to provide clinical insights for […]
The Search for Answers About Aplasia Cutis Congenita
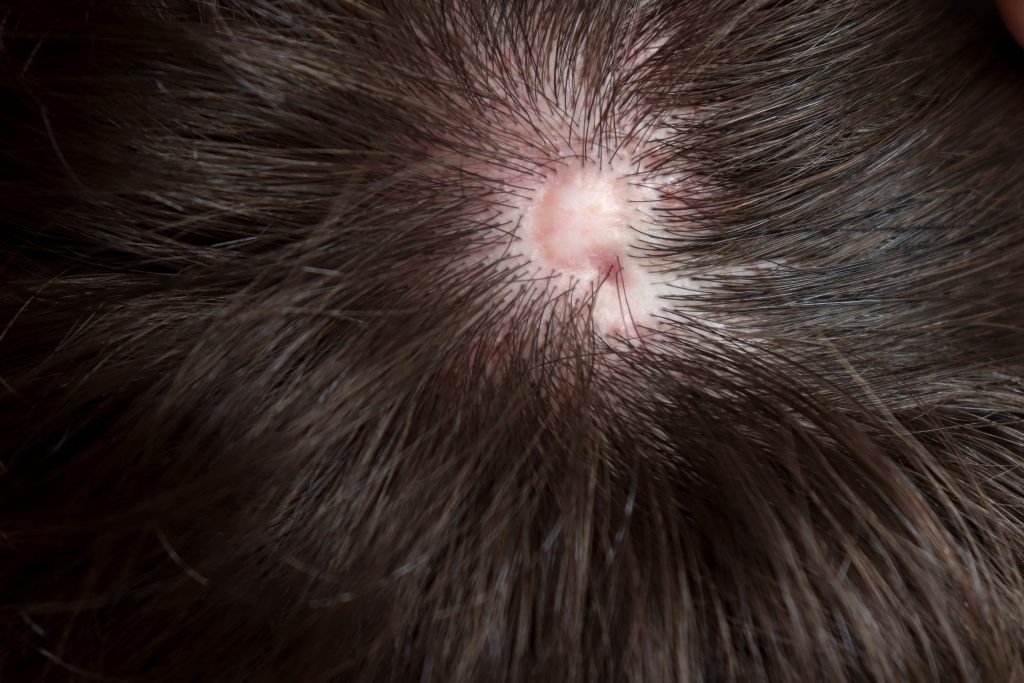
Aplasia cutis congenita is a congenital epidermal defect of the midline scalp, but why it forms mainly at this anatomic site has been unknown. Now, researchers have now identified the mechanisms behind mutations that cause a congenital condition called aplasia cutis congenita. Depending on whether mutations are in the KCTD1 or KCTD15 genes, additional characteristics beyond the scalp—such as kidney […]
