Quest Diagnostics Rolls Out New Gene Expression Test for Melanoma risk Assessment
Quest Diagnostics is rolling out MelaNodal Predict, a predictive gene expression test that can help personalize treatment decisions melanoma patients. Dermatologists and other providers nationwide may begin ordering the test today from Quest Diagnostics, as well as through Dermpath Diagnostics, Quest’s subspecialty pathology business. MelaNodal Predict is based on technology developed by SkylineDx and the Mayo […]
Introducing TDD’s New Editorial Advisory Board

Drum roll, please…The Derm Digest is proud to announce the newest members of its Editorial Advisory Board. Joining the inaugural editorial board are… Christopher Bunick, MD, PhD Dr. Bunick is an associate professor of dermatology at Yale University in New Haven, CT. Raj Chovatiya, MD, PhD Dr. Chovatiya is the Founder and Director of the […]
Non-Lesional Filaggrin Expression May Predict Asthma in Kids With AD

Low filaggrin (FLG) expression in children aged 2 to 3 may predict of school-age asthma, according to new research slated to be presented at the 2024 American Academy of Allergy, Asthma & Immunology Annual Meeting, in Washington, DC. In the study, pulmonary function testing (PFT) was conducted in 96 children, aged 7 to 8 years old, […]
First Patient Dosed in Artax’s Phase 2a Psoriasis Trial Evaluating AX-158
The first patient has been dosed in the Artax’s Phase 2a trial evaluating the safety and biomarker responses of AX-158 in a first proof of mechanism trial in psoriasis. AX-158 has potential to be the first immunomodulator in the Nck blocker class, the Company states. Safety and efficacy results are expected in second half of 2024. “The new Nck […]
FDA, EMA Accept Galderma’s BLA for Nemolizumab for PN and AD

The U.S. Food and Drug Administration (FDA) has accepted Galderma’s Biologics License Applications for nemolizumab for the treatment of patients with prurigo nodularis (PN) and for adolescents and adults with moderate to severe atopic dermatitis (AD). In addition, the European Medicines Agency has also accepted the Marketing Authorization Applications for nemolizumab in prurigo nodularis and […]
Dermavant Submits Supplemental sNDA to FDA for VTAMA Cream for AD in Adults and Children 2 Years of Age and Older
Dermavant Sciences has submitted a Supplemental New Drug Application (sNDA) to the U.S. Food and Drug Administration (FDA) for VTAMA (tapinarof) cream, 1% for the topical treatment of atopic dermatitis (AD) in adults and children 2 years of age and older. “We are confident, that VTAMA cream, if approved by the FDA, will be well […]
Head-to-Head Study: EscharEx Bests Santyl for Treating VLUs
MediWound Ltd’s EscharEx bested market leader collagenase Santyl ointment in wound debridement, promotion of granulation tissue, and time to wound closure in patients with chronic venous leg ulcers (VLU). EscharEx is a bioactive, multimodal debridement therapy for the treatment of chronic and other hard-to-heal wounds that is currently in the advanced stages of clinical development. […]
New Thinking About Non-melanoma Skin Cancer Treatment
Checkpoint inhibitors have changed the way that many types of cancer are treated, and these powerful immunotherapy drugs may also have a role in basal cell carcinoma and squamous cell carcinoma, according to Andrew Weinstein, MD, a dermatologist in Boynton Beach, FL.
Is a Pharmacologic Treatment for Neurofibromatosis Type 1 Skin Tumors on the Horizon?
Current treatment for neurofibromatosis type 1 skin tumors involves monitoring the tumors and removing them surgically if necessary, but this isn’t ideal as neurofibromas can grow back and continue originating in other parts of the body, plus surgery leaves scars. Now, scientists have identified a key player in the protein signature: the GPR68 receptor. When it is activated […]
Barzolvolimab Shows Promise in CSU
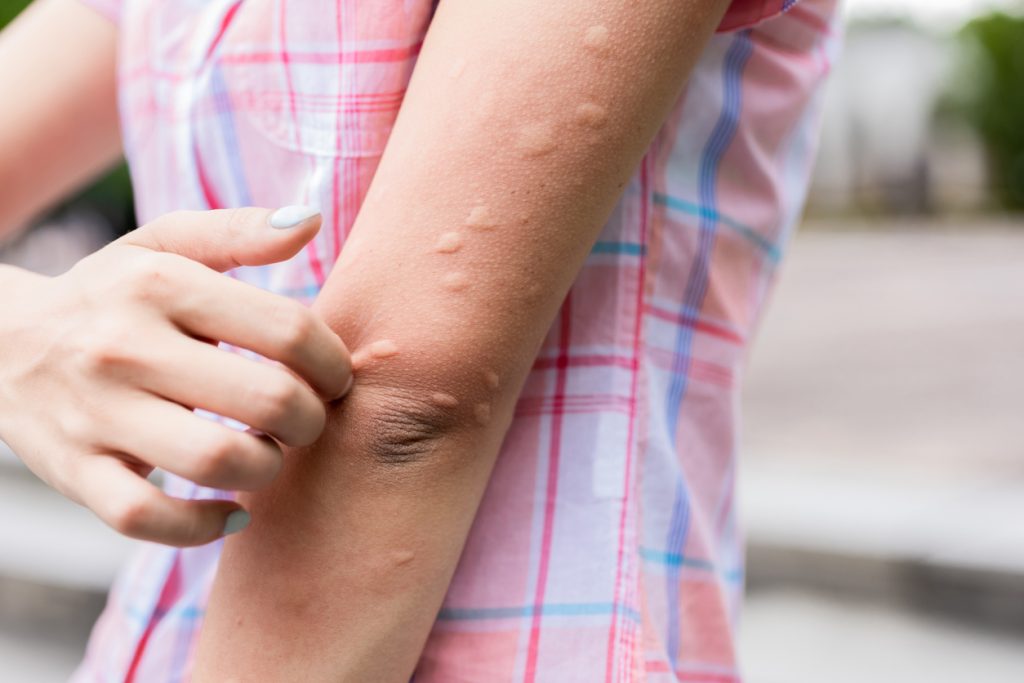
Chronic spontaneous urticaria (CSU) is getting a lot of attention these days as researchers close in on the role mast cells play in causing hives. Top-line results from a phase 2 trial show that barzolvolimab (anti-KIT monoclonal antibody) improves symptoms in patients with antihistamine-refractory CSU. “Barzolvolimab successfully decreased disease activity in patients with moderate to […]
