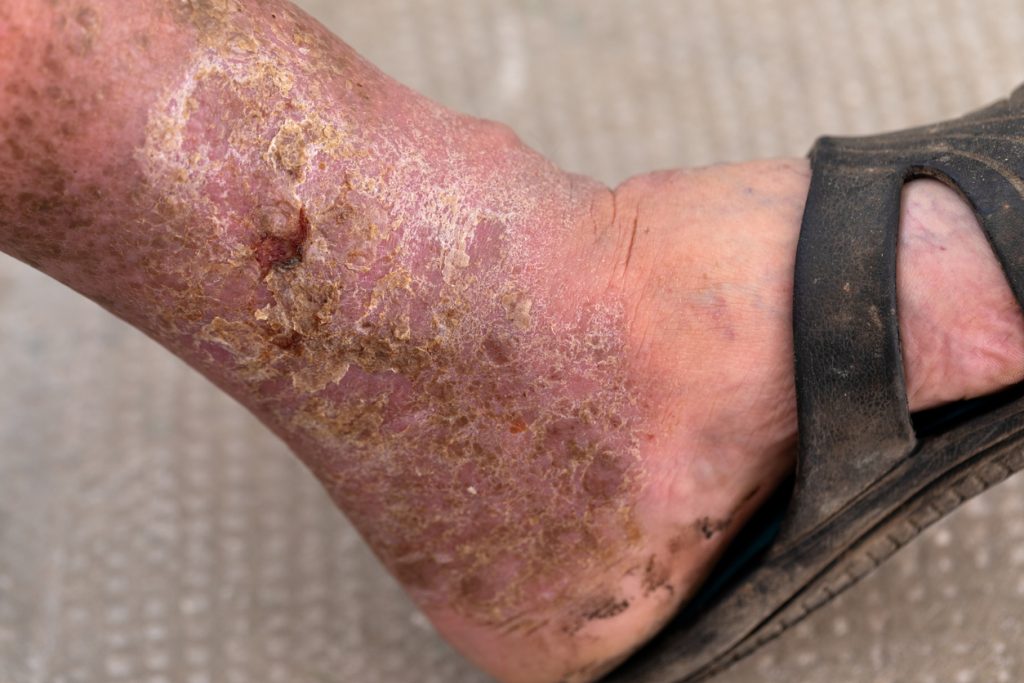
MediWound Ltd’s EscharEx bested market leader collagenase Santyl ointment in wound debridement, promotion of granulation tissue, and time to wound closure in patients with chronic venous leg ulcers (VLU).
EscharEx is a bioactive, multimodal debridement therapy for the treatment of chronic and other hard-to-heal wounds that is currently in the advanced stages of clinical development. It is a concentrate of proteolytic enzymes enriched in bromelain for topical, easy to use daily applications
Results from the previously disclosed Phase II study (ChronEx), which evaluated the safety and efficacy of EscharEx, demonstrated the superiority of EscahrEx vs. a gel vehicle (placebo) and non-surgical standard of care (NSSOC) in achieving complete debridement of non-viable tissue and promotion of granulation tissue.
Now, the secondary analyses assessed the incidence and time to complete debridement, complete granulation, and wound closure in patients treated with EscharEx (n=46) compared to a sub-group of patients who were treated with Santyl (n=8).
Baseline characteristics (age, gender, wound age, wound size) were comparable in both groups. The incidence of complete debridement during the daily treatment period (the first two weeks of the study) was 63.0% (95% CI=47.5-76.8) for EscharEx vs. 0% for Santyl. The estimated median time to achieve complete debridement during the study was nine days for EscharEx vs. not achieved for Santyl.
The incidence of achieving complete debridement and complete cover of the wound bed with granulation tissue during the daily treatment period was 50% for EscharEx vs. 0% for Santyl.
The incidence of achieving wound bed preparation (WBP) throughout the study was 78.3% for EscharEx vs. 37.5% for Santyl. The estimated median time to achieve WBP was 11 days for EscharEx vs. not achieved for Santyl. Fifteen of the 46 patients (32.6%) treated with EscharEx completely closed their wounds during the study, compared to two out of eight patients (25%) treated with Santyl. In those patients who achieved complete wound closure, the average time to wound closure was 48.4 days for EscharEx vs. 76.0 days for Santyl. Patient reported applicational pain was comparable in both groups. The safety profile and overall incidence of adverse wound reactions were comparable between arms.
“Complete debridement and complete granulation are key components of wound bed preparation, a critical step in the transition of a chronic wound from an abnormal, disrupted healing process to a normal healing process,” says Dr. Robert J. Snyder, Chief Medical Officer of MediWound, in a news release. “These results further support the potential superiority of EscharEx compared with Santyl in both the percentage of wounds achieving these critical steps, as well as the timeframe in which they are achieved. These significant differences could have a profound impact on wound healing, prevention of complications, and reduction in disease burden.”
“These head-to-head results position EscharEx to become the market leader in enzymatic agents for the treatment of chronic wounds,” adds Ofer Gonen, CEO of MediWound. “Data from clinical studies show that EscharEx provides a multimodal mechanism of action for debridement and promotion of granulation tissue, as well as reduction of biofilm and bioburden. All are achieved within a short time frame to facilitate early wound closure, a major benefit for patients suffering from chronic non-healing wounds. With such promising Phase II data, we look forward to the upcoming Phase III trial, set to begin in the second half of 2024, as planned.”
The data is scheduled for presentation in May 2024 at The Wound Healing Society, the Symposium on Advanced Wound Care, and the European Wound Management Association.