Current treatment for neurofibromatosis type 1 skin tumors involves monitoring the tumors and removing them surgically if necessary, but this isn’t ideal as neurofibromas can grow back and continue originating in other parts of the body, plus surgery leaves scars.
Now, scientists have identified a key player in the protein signature: the GPR68 receptor. When it is activated by a molecule named Ogerin and combined with Selumetinib, a MEK inhibitor already approved for use in plexiform neurofibromas, this receptor triggers a notable decrease in the survival of the primary Schwann cells derived from cutaneous neurofibromas.
In the study, researchers have explored how Schwann cells and fibroblasts interact with each other in cutaneous neurofibromas, revealing a gene signature indicative of their communication. This signature supports that their interplay may be a driving factor in the growth of cutaneous neurofibromas.
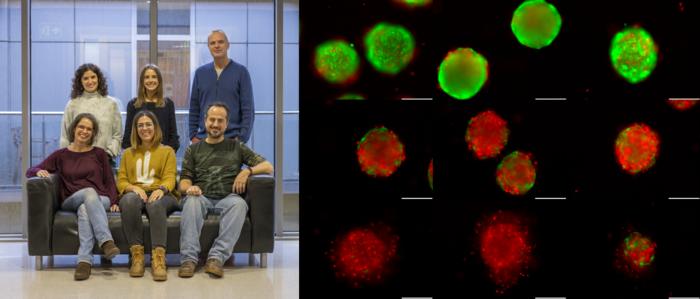
Essentially, this combination not only halts the proliferation of Schwann cells but also promotes their terminal differentiation and eventually leads to their death. This significant outcome has been further validated using a sophisticated 3D model of neurofibromas developed from induced pluripotent stem cells.
The effect is not limited to the Ogerin-Selumetinib combination. The researchers have observed similar results when they used other substances that activate GPR68 or mimic the action of a cellular messenger known as cAMP.
The key takeaway from these experiments is the lasting impact of the treatment. While Selumetinib on its own only temporarily pauses the proliferation of Schwann cells, the combination with Ogerin achieves an enduring halt in their expansion, maintaining this effect even after the treatment has ended.
These results achieved in vitro suggest a new approach to treating cutaneous neurofibromas. By disrupting the balance of two critical cellular pathways – the Ras pathway (affected by MEK inhibitors like Selumetinib) and the cAMP pathway (targeted by cAMP elevators like Ogerin) – this combination therapy emerges as a promising strategy. This could potentially shift the current treatment paradigm for neurofibromatosis type 1 skin tumors from surgery to a more targeted pharmaceutical approach.
“In this work, we have identified the GPR68 receptor and analyzed the effect of one of its activators. We have also demonstrated that, in fact, any treatment that provides an increase in cAMP in the cells responsible for tumor growth can be valid,” says study author Helena Mazuelas of the Germans Trias i Pujol Research Institute (IGTP) Hereditary Cancer group in Barcelona, Spain, in a news release. “Many drugs would perform this function, many of which are already being tested in humans for other diseases not related to neurofibromatosis type 1, and humans for other diseases not related to neurofibromatosis type 1, and which could potentially also work for neurofibromas. We are looking for them.”
The findings appear in JCI Insight.