FDA Approves Topical for Tuberous Sclerosis Complex
By Ted Rosen, MD, FAAD

Tuberous sclerosis complex (TSC) is an autosomal dominant genetic disorder that causes various types of tumors in a number of different tissues, including the skin. Some 50,000 individuals suffer from TSC in the United States.
Aside from tumor growth, TSC has been associated with autism, intellectual disability, and seizures. From 75% to 80% of TSC patients, regardless of race, gender, or ethnicity, will eventually develop facial angiofibromas due to unregulated tissue growth. Specifically, these lesions result from persistent and aberrant activation of the mammalian target of rapamycin (mTOR) pathway.
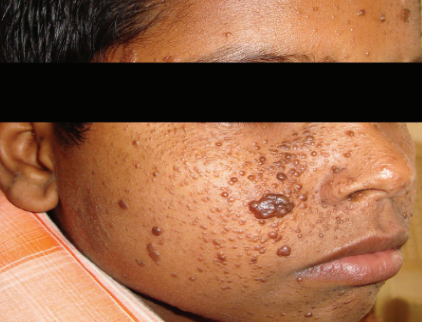
Morphologically, facial angiofibromas present as reddish papules on the cheeks, nose, and chin. (See Figure 1) Without therapy, these lesions can range from mildly cosmetically distressing to grossly disfiguring, and they may bleed and/or itch.
Previously, the only therapeutic interventions for facial angiofibroma consisted of costly surgical or ablative procedures, which also ran the risk of dyschromia and scarring.
The FDA recently approved sirolimus topical gel (Hyftor, Nobelpharma) for facial angiofibromas associated with TSC. This agent is approved for use in both adults and children over the age of 6. The active ingredient, sirolimus, works by inhibiting mTOR, a serine/threonine kinase which belongs to a series of related kinases responsible for cellular growth and proliferation.
Clinical trial results were clearly positive, though not overwhelming. Approval was based upon a single randomized, double-blind, vehicle-controlled study (NCT02635789) involving 62 patients. Subjects applied either sirolimus 0.2% gel or vehicle twice daily for 12 weeks. The primary endpoint was a composite improvement in both redness and size of facial angiofibromas compared to baseline. “Improved” was defined as a 50% reduction in size and a 2-level reduction in redness. “Markedly improved” was defined as a 75% reduction in size and a 3-level reduction in erythema.
At the end of the trial, 23% of sirolimus-treated patients were “improved” or “markedly improved.” By comparison, only 6% of the vehicle-treated patients were judged “improved” or “markedly improved.”
The theoretical risks of Hyftor use include elevation of cholesterol and triglycerides, reduced efficacy of vaccines, fetal harm when administered during pregnancy, development of lymphoma and other malignancies, and serious infections. These risks, however, are actually predicated on the possible adverse events associated with oral sirolimus administration. Sirolimus is a photosensitizer, so appropriate precautions should be taken with regard to sun exposure. Actual side effects observed during the pivotal trial included: dry skin, irritation, itching, and bleeding at the sites of application, as well as acne, acneiform dermatitis, and ocular hyperemia.
The drug will be supplied in a 10-gram tube containing 2 mg of sirolimus per gram. It should be refrigerated. The product will be available in the very near future.
REFERENCE
Hyftor package insert, 2022. https://hyftorpi.com/wp-content/uploads/2022/04/HYFTOR_Approved-PI-PPI_3.22-1-2.pdf. Accessed April 25, 2022.