The authors discuss their experience with a young adult alopecia areata patient who, despite being on several medical therapies, continued to get worse. They call the addition of upadacitinib 15 mg daily a “therapeutic triumph.”
Upadacitinib for Severe Alopecia Areata
By Emily Hall, DNPCa; Margaret DiRuggiero, BSb; Douglas DiRuggiero, DMSc, PA-Ca; and John Chung, MDa
a. Skin Cancer and Cosmetic Dermatology, Dalton and Rome, Georgia
b. United States Air Force Academy, Colorado Springs, Colorado
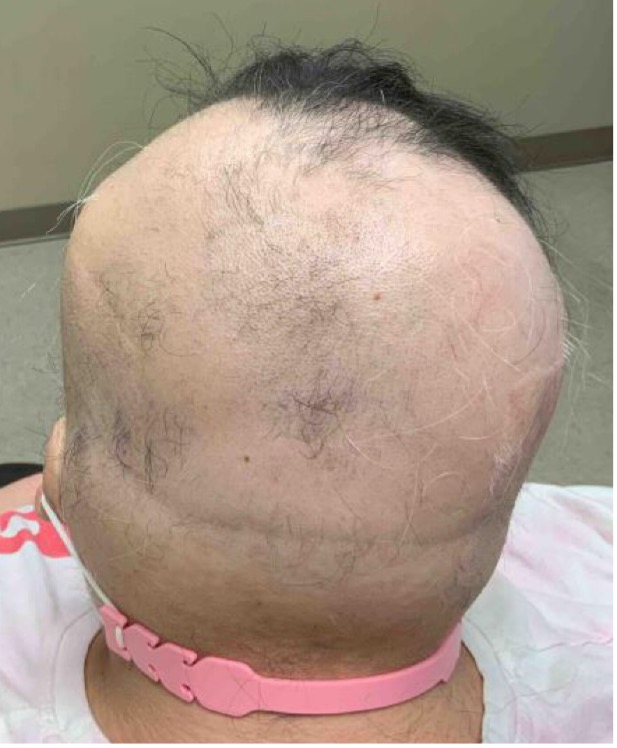
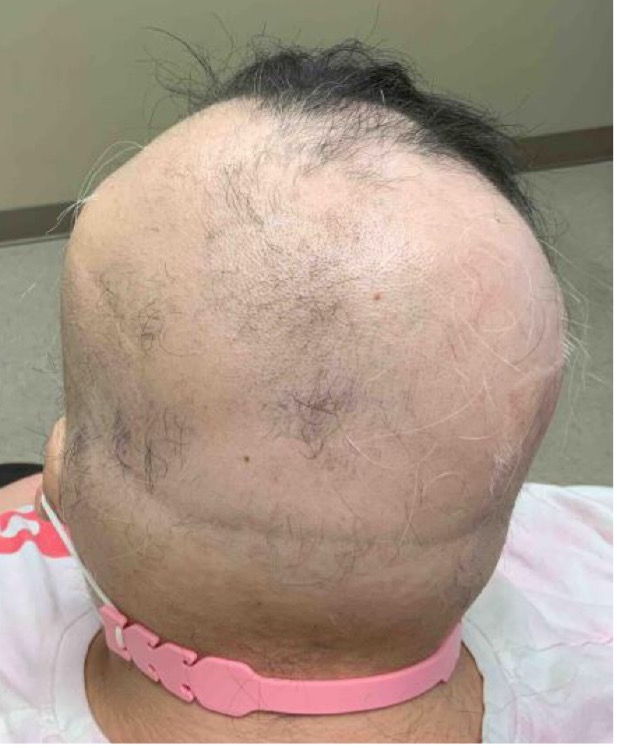
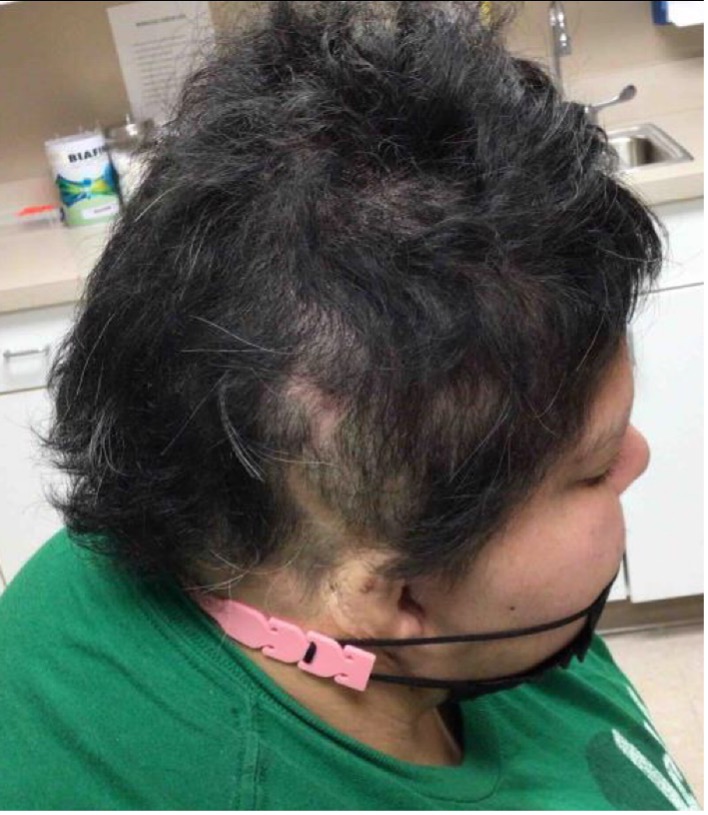
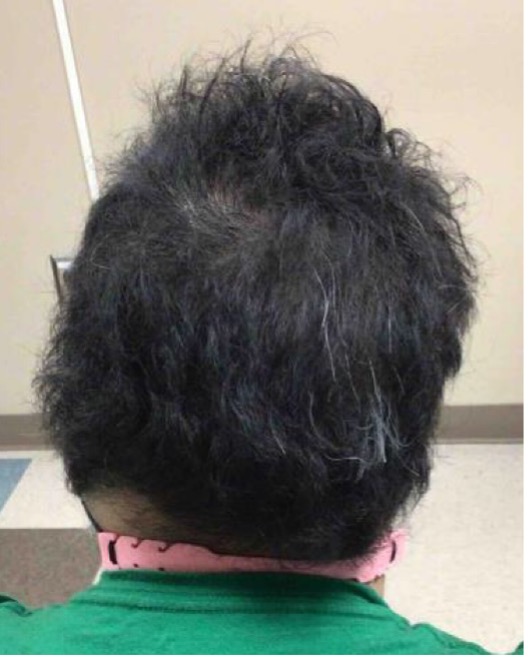
An established 20-year-old female patient with continuous and progressive alopecia areata (AA) for 8 years recently presented to the clinic for reevaluation. Despite oral, intralesional, and topical steroids, as well as topical minoxidil, her alopecia worsened to a Severity of Alopecia Tool (SALT) score of 71% total: 18% left scalp, 13.5% right scalp, 20% top of scalp, and 20% back of scalp; and with no eyebrows (see Figures 1-A,B,C).
Upadacitinib (RINVOQ; Abbvie) 15 mg daily was added to her regimen.
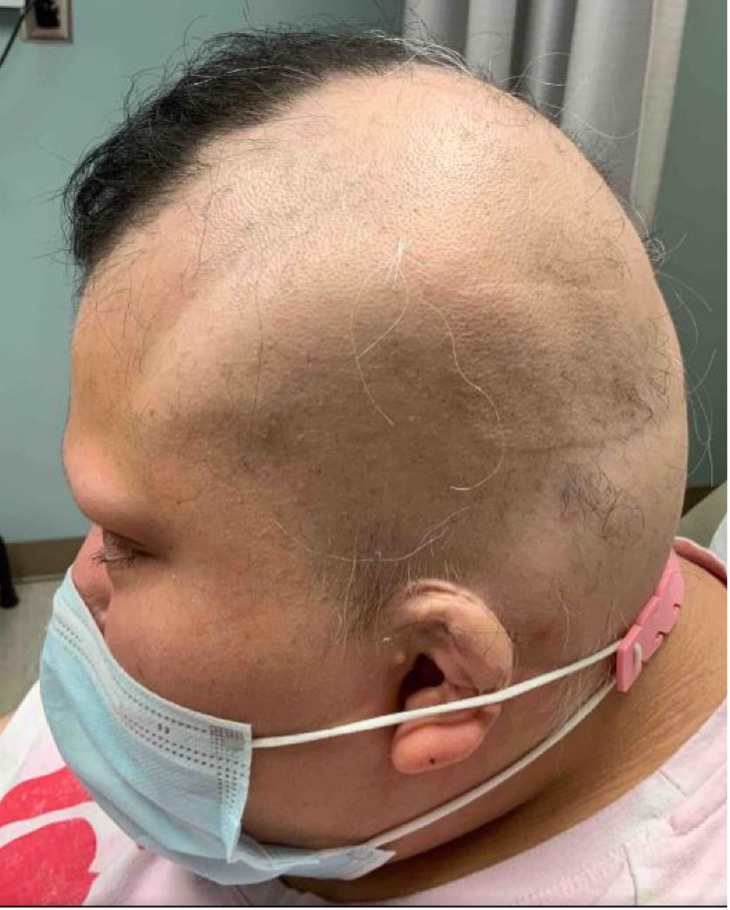
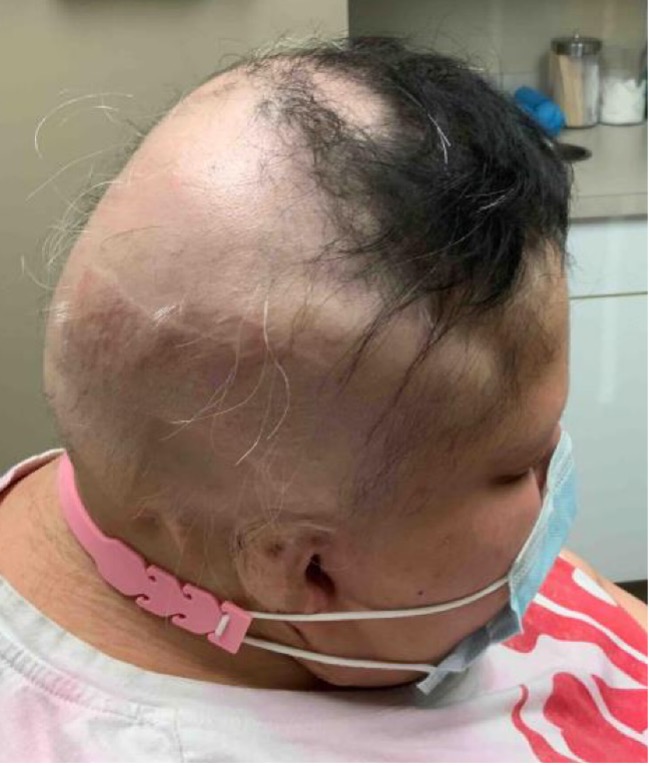
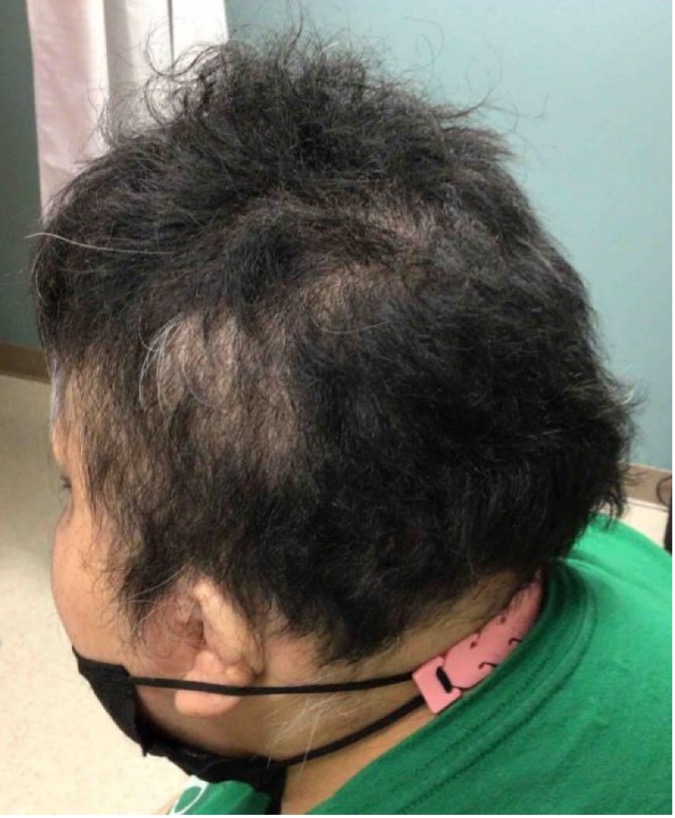
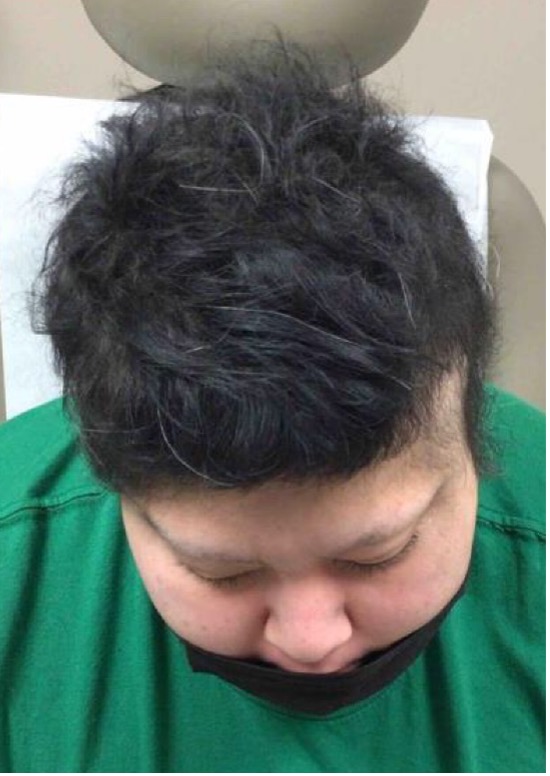
Six months later, her total SALT score decreased to 8% (see Figures 2-A,B,C,D). This is truly a remarkable therapeutic triumph.
From a dermatologic perspective, it should be noted that upadacitinib received FDA approval for treatment of moderate-to-severe atopic dermatitis in people aged 12 and older in February 2021. However, due to the broad spectrum of immunologic effects associated with the various Janus kinase (JAK) inhibitors, it is very likely that they will prove beneficial in a wide variety of dermatologic disorders.
Alopecia areata is a complex autoimmune illness that affects approximately 2% of the population, with the majority of cases appearing in middle age.1 Several of the cytokines that appear to be involved in the pathophysiology of alopecia areata are required in JAK-STAT signaling such as interleukin (IL)-2, IL-7, IL-15, IL-21, and interferon-gamma (IFN-g), thus making this pathway promising as a therapeutic target.1
Currently, baricitinib—a different JAK inhibitor—is the first and only FDA-approved treatment for alopecia areata. Nearly 39% of patients with severe alopecia, treated with baricitinib 4 mg daily, achieved a SALT score of 20 or less at 36 weeks (n = 654; BRAVE-AA1 and BRAVE-AA2).2
Upadacitinib has also been reported to improve chronic alopecia areata,3 but is not currently FDA approved for this specific disease state.
While the patient in this case presentation was still using oral steroids and other topical treatments, those medications were being maintained at stable doses despite worsening of her AA. The only change in her therapeutic regimen was the addition of upadacitinib 15 mg daily. She remains on upadacitinib 15 mg daily and her hair remains full nearly a year after initiation. It is uncertain what might happen if the JAK inhibitor dose were to be lowered or the medication stopped.
References:
- Ali E, Owais R, Sheikh A, et al. Olumniant (Baricitinib) oral tablets: An insight into FDA-approved systemic treatment for Alopecia Areata. Ann Med Surg (Lond). 2022 Jul 13;80:104157. doi: 10.1016/j.amsu.2022.104157. PMID: 36045780; PMCID: PMC9422172.
- King B, Ohyama M, Kwon O, et al. Two Phase 3 Trials of Baricitinib for Alopecia Areata. N Engl J Med. 2022 May 5;386(18):1687-1699. doi: 10.1056/NEJMoa2110343. Epub 2022 Mar 26. PMID: 35334197.
- Asfour L, Getsos Colla T, Moussa A, et al. Concurrent chronic alopecia areata and severe atopic dermatitis successfully treated with upadacitinib. Int J Dermatol. 2022 Nov;61(11):e416-e417. doi: 10.1111/ijd.16316. Epub 2022 Jun 20. PMID: 35722925.
Disclosures: Douglas DiRuggiero is a speaker/advisory board member for Amgen, Abbvie, Arcutis, Bristol-Myers-Squibb, Epi Health, Incyte, Lilly, Novartis, Regeneron, Sanofi, Sun Pharmaceuticals, and UCB. Margaret DiRuggiero, Emily Hall, and Dr. John Chungreport no relevant financial interests.