Tapinarof Cream for Psoriasis
Novel agent shows promise for expanding topical treatment options
With Linda Stein Gold, MD

The New Drug Application for tapinarof 1% cream (Dermavant Sciences) for treatment of mild-to-severe plaque psoriasis is undergoing FDA review. If approved, the novel nonsteroidal agent will be an important addition to the dermatologist’s treatment armamentarium, according to Linda Stein Gold, MD.
“Head-to-head comparison trials are needed to understand how tapinarof compares with existing treatments for plaque psoriasis, but I am impressed by the efficacy and tolerability that we found for tapinarof cream in the randomized, double-blind, vehicle-controlled phase 3 trials,” said Dr. Stein Gold, who was a study investigator.
“After 12 weeks of treatment, about 40% of patients using tapinarof cream once daily achieved a Physician Global Assessment (PGA) score of clear (0) or almost clear (1) with a ≥2-grade improvement from baseline. And data from the 40-week, long-term extension study showed an impressive remittive effect and continued safety. Patients who achieved clear skin while on tapinarof in the double-blind study maintained clear or almost clear skin for an average of 4 months after stopping tapinarof, and the safety profile of tapinarof among patients who continued treatment was consistent with that observed with shorter term use.”
First-in-class therapeutic
Tapinarof is an aryl hydrocarbon receptor modulator thought to be effective in clearing psoriasis through a multimodal mechanism of action that includes immune modulation, normalization of the skin barrier, and antioxidant effects.
“The aryl hydrocarbon receptor modulates the expression of type 17 helper T cytokines that are implicated in the pathogenesis of psoriasis, including interleukin-17A and interleukin-17F. It also mediates expression of filaggrin and loricrin and expression of genes for enzymes with antioxidant activity,” explained Dr. Stein Gold, Director of Dermatology Clinical Research and Head, Division of Dermatology, Henry Ford Health System, Detroit, Michigan.
Pivotal PSOARING trials
Results from the two pivotal trials investigating tapinarof cream for treatment of plaque psoriasis were published in the New England Journal of Medicine in December 2021.1 Known as PSOARING 1 and PSOARING 2, the identically designed trials were conducted in the U.S. and Canada. They enrolled adults ages 18 to 75 years with a ≥6-month history of stable psoriasis, a baseline PGA score of 2 (mild) to 4 (severe), and total body surface area (BSA) involvement of 3% to 20% excluding the scalp, palms, fingernails, toenails, and soles. Patients underwent washout of existing treatments for psoriasis except for non-medicated emollients and were stratified at enrollment according to baseline PGA. Per protocol, patients with mild and severe psoriasis could each only represent 10% of the total enrollment.
Participants were randomized 2:1 to treatment with tapinarof or vehicle cream once daily for 12 weeks. The instructions for use were to apply the assigned product to all lesions and to continue application even as sites improved or cleared.
The two trials enrolled a total of 1025 patients. Their mean baseline PASI score was about 9.0 and mean percent BSA of involvement was almost 8%. A majority of patients had psoriasis for >10 years.
The percentage of patients achieving the primary efficacy endpoint (PGA score 0 or 1 and ≥2-point improvement) in the tapinarof groups was slightly higher in PSOARING 2 than in PSOARING 1 (40% vs 35.4%). Only about 6% of patients in the vehicle cream group in both studies achieved the primary end point (P <.0001 vs tapinarof).
Secondary efficacy analyses of PASI 75 and PASI 90 responder rates, percentages of patients achieving a PGA score of 0 or 1, and mean change in % affected BSA also showed statistically significant differences favoring tapinarof (Table). Data from patient-reported outcomes that included Peak Pruritus Numeric Rating Scale scores and Dermatology Life Quality Index scores were not analyzed for statistical significance but showed numerical differences favoring tapinarof.
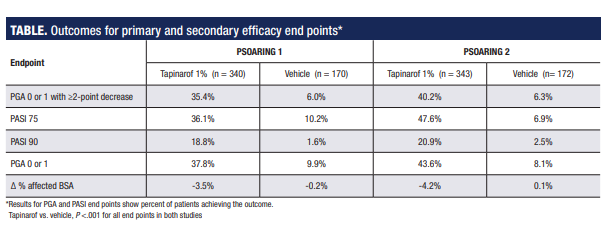
*Results for PGA and PASI endpoints show the percent of patients achieving the outcome.Tapinarof vs. vehicle, P <.001 for all endpoints in both studies
Perspective on safety
Folliculitis was the most common adverse event associated with tapinarof with an overall rate of approximately 20% versus <1% for vehicle. However, the risk for folliculitis is not an issue that would limit tapinarof use, said Dr. Stein Gold.
“With the exception of a single patient, the folliculitis cases in the tapinarof groups were graded mild to moderate in severity. Furthermore, I think it is important to look at the related dropout rate for insight as to how significant any particular adverse event is to patients. In PSOARING 1 and PSOARING 2, the percentage of patients who discontinued study participation because of folliculitis was only 1.8% and 0.9%, respectively.”
Other than folliculitis, contact dermatitis was the only dermatologic adverse event that occurred at an overall rate >5%, and it was associated with study discontinuation rates of 1.5% and 2.0% in the two trials. Headache occurred in the tapinarof and vehicle groups at rates of 3.8% and 2.4%, respectively, in PSOARING 1, and 3.8% and 0.6%, respectively, in PSOARING 2. There was no evidence of adverse systemic effects associated with tapinarof.
“Importantly, patient reports showed that any burning, stinging, or itching associated with tapinarof was mild on average and milder than in the vehicle group, while investigator ratings showed application site irritation was minimal at worst,” said Dr. Stein Gold.
By Cheryl Guttman Krader
____________________________________________________________________________________________________
Reference
1. Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385(24):2219-2229. DOI: 10.1056/NEJMoa2103629.
________________________________________________________________________________________________________________
Disclosures
Dr. Stein Gold is a consultant to and investigator for Dermavant Sciences.